Eicosanoid

Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a sub-category of oxylipins, i.e. oxidized fatty acids of diverse carbon units in length, and are distinguished from other oxylipins by their overwhelming importance as cell signaling molecules. Eicosanoids function in diverse physiological systems and pathological processes such as: mounting or inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. In performing these roles, eicosanoids most often act as autocrine signaling agents to impact their cells of origin or as paracrine signaling agents to impact cells in the proximity of their cells of origin. Some eicosanoids, such as prostaglandins, may also have endocrine roles as hormones to influence the function of distant cells.[1][2]
There are multiple subfamilies of eicosanoids, including most prominently the prostaglandins, thromboxanes, leukotrienes, lipoxins, resolvins, and eoxins.[1] For each subfamily, there is the potential to have at least 4 separate series of metabolites, two series derived from the ω−6 PUFAs arachidonic and dihomo-gamma-linolenic acids, one series derived from the ω−3 PUFA eicosapentaenoic acid, and one series derived from the ω−9 PUFA mead acid. This subfamily distinction is important. Mammals, including humans, are unable to convert ω−6 into ω−3 PUFA. In consequence, tissue levels of the ω−6 and ω−3 PUFAs and their corresponding eicosanoid metabolites link directly to the amount of dietary ω−6 versus ω−3 PUFAs consumed.[3] Since certain of the ω−6 and ω−3 PUFA series of metabolites have almost diametrically opposing physiological and pathological activities, it has often been suggested that the deleterious consequences associated with the consumption of ω−6 PUFA-rich diets reflects excessive production and activities of ω−6 PUFA-derived eicosanoids, while the beneficial effects associated with the consumption of ω−3 PUFA-rich diets reflect the excessive production and activities of ω−3 PUFA-derived eicosanoids.[4][5][6][7] In this view, the opposing effects of ω−6 PUFA-derived and ω−3 PUFA-derived eicosanoids on key target cells underlie the detrimental and beneficial effects of ω−6 and ω−3 PUFA-rich diets on inflammation and allergy reactions, atherosclerosis, hypertension, cancer growth, and a host of other processes.
Nomenclature
[edit]Fatty acid sources
[edit]"Eicosanoid" (from Greek eicosa- 'twenty') is the collective term[8] for straight-chain PUFAs (polyunsaturated fatty acids) of 20 carbon units in length that have been metabolized or otherwise converted to oxygen-containing products. The PUFA precursors to the eicosanoids include:
- Arachidonic acid (AA), i.e. 5Z,8Z,11Z,14Z-eicosatetraenoic acid is an ω−6 fatty acid with four double bonds in the cis configuration (denoted Z in E–Z notation), each located between carbons 5-6, 8-9, 11-12, and 14-15 (see carbon numbering).
- Adrenic acid (AdA), i.e. 7Z,10Z,13Z,16Z-docosatetraenoic acid, is an ω−6 fatty acid with four cis double bonds, each located between carbons 7-8, 10-11, 13-14, and 16-17.
- Eicosapentaenoic acid (EPA), i.e. 5Z,8Z,11Z,14Z,17Z-eicosapentaenoic acid is an ω−3 fatty acid with five cis double bonds, each located between carbons 5-6, 8-9, 11-12, 14-15, and 17-18.
- Dihomo-gamma-linolenic acid (DGLA), i.e. 8Z,11Z,14Z-eicosatrienoic acid is an ω−6 fatty acid with three cis double bonds, each located between carbons 8-9, 11-12, and 14-15.
- Mead acid, i.e. 5Z,8Z,11Z-eicosatrienoic acid, is an ω−9 fatty acid containing three cis double bonds, each located between carbons 5-6, 8-9, and 11-12.
Abbreviation
[edit]A particular eicosanoid is denoted by a four-character abbreviation, composed of:
- its two-letter abbreviation (LT, EX or PG, as described below),[9]
- one A-B-C sequence-letter,[10]
- A subscript or plain script number following the designated eicosanoid's trivial name indicates the number of its double bonds. Examples are:
- The EPA-derived prostanoids have three double bonds (e.g. PGG3 or PGG3) while leukotrienes derived from EPA have five double bonds (e.g. LTB5 or LTB5).
- The AA-derived prostanoids have two double bonds (e.g. PGG2 or PGG2) while their AA-derived leukotrienes have four double bonds (e.g. LTB4 or LTB4).
- Hydroperoxy-, hydroxyl-, and oxo-eicosanoids possess a hydroperoxy (-OOH), hydroxy (-OH), or oxygen atom (=O) substituents link to a PUFA carbon by a single (-) or double (=) bond. Their trivial names indicate the substituent as: Hp or HP for a hydroperoxy residue (e.g. 5-hydroperooxy-eicosatraenoic acid or 5-HpETE or 5-HPETE); H for a hydroxy residue (e.g. 5-hydroxy-eicosatetraenoic acid or 5-HETE); and oxo- for an oxo residue (e.g. 5-oxo-eicosatetraenioic acid or 5-oxo-ETE or 5-oxoETE). The number of their double bonds is indicated by their full and trivial names: AA-derived hydroxy metabolites have four (i.e. 'tetra' or 'T') double bonds (e.g. 5-hydroxy-eicosatetraenoic acid or 5-HETE; EPA-derived hydroxy metabolites have five ('penta' or 'P') double bonds (e.g. 5-hydroxy-eicosapentaenoic acid or 5-HEPE); and DGLA-derived hydroxy metabolites have three ('tri' or 'Tr') double bonds (e.g. 5-hydroxy-eicosatrienoic acid or 5-HETrE).
The stereochemistry of the eicosanoid products formed may differ among the pathways. For prostaglandins, this is often indicated by Greek letters (e.g. PGF2α versus PGF2β). For hydroperoxy and hydroxy eicosanoids an S or R designates the chirality of their substituents (e.g. 5S-hydroxy-eicosateteraenoic acid [also termed 5(S)-, 5S-hydroxy-, and 5(S)-hydroxy-eicosatetraenoic acid] is given the trivial names of 5S-HETE, 5(S)-HETE, 5S-HETE, or 5(S)-HETE). Since eicosanoid-forming enzymes commonly make S isomer products either with marked preference or essentially exclusively, the use of S/R designations has often been dropped (e.g. 5S-HETE is 5-HETE). Nonetheless, certain eicosanoid-forming pathways do form R isomers and their S versus R isomeric products can exhibit dramatically different biological activities.[11] Failing to specify S/R isomers can be misleading. Here, all hydroperoxy and hydroxy substituents have the S configuration unless noted otherwise.
Classic eicosanoids
[edit]Current usage limits the term eicosanoid to:
- ω−6 series eicosanoids derived from arachidonic acid:
- Hydroxyeicosatetraenoic acids (HETE) include the following metabolites of arachidonic acid:
- 5-HETE, 12-HETE, 15-hydroxyeicosatetraenoic acid (i.e. 15-HETE), 20-hydroxyeicosatetraenoic acid (i.e. 20-HETE), and 19-HETE.
- Leukotrienes (LT) include the following metabolites of arachidonic acid:
- Eoxins (EX) include the following metabolites of arachidnoic acid:
- Prostanoids consisting of several different types:
- Prostaglandins (PG) include the following metabolites of arachidonic acid:
- Prostacyclins include:
- PGI2.
- Thromboxanes (TX) include the following metabolites of arachidonic acid:
- Cyclopentenone prostaglandins include the following metabolites of arachidonic acid:
- PGA1, PGA2 (see Prostanoid, PGJ2, Δ12-PGJ2, and 15-deoxy-Δ12,14-PGJ2).[12]
- Hydroxyeicosatetraenoic acids (HETE) include the following metabolites of arachidonic acid:
- ω−6 series eicosanoids derived from dihomo-gamma-linolenic acid. These metabolites are analogs of arachidonic acid-derived eicosanoids but lack a double bond between carbons 5 and 6 and therefore have 1 less double bond than their arachidonic acid-derived analogs. They are the following:
- ω−3 series eicosanoids:
- Resolvins of the E series (RvE) (D series resolvins [RvD] are metabolites of the 22-carbon ω−3 fatty acid docosahexaenoic acid; see Specialized pro-resolving mediators § DHA-derived resolvins). RvE include the following metabolites of eicosapentaenoic acid:
- RvE1, 18S-RvE1, RvE2, and RvE3.
- Other ω−3 series eicosapentaenoic acid-derived eicosanoids are analogs of ω−6 fatty acid-derived metabolites but contain a double bond between carbon 17 and 18 and therefore have one more double bond than their arachidonic acid-derived analogs. They include (HEPE is hydroxyeicosapentaenoic acid):
- 5-HEPE, 12-HEPE,[15] 15-HEPE,[16] and 20-HETE;[17] LTA5, LTB5, LTC5, LTD5, and LTE5 (see Arachidonate 5-lipoxygenase § Eicosapentaenoic acid);[18] PGE3, PGD3, PGF3α, and Δ(17)-6-keto PGF1α;[18][19] PGI3 (see Essential fatty acid interactions § Counteraction);[18] and TXA3 and TXB3.[18]
- Resolvins of the E series (RvE) (D series resolvins [RvD] are metabolites of the 22-carbon ω−3 fatty acid docosahexaenoic acid; see Specialized pro-resolving mediators § DHA-derived resolvins). RvE include the following metabolites of eicosapentaenoic acid:
- ω−9 series eicosanoids
- Hydroxy are derived form mead acid, is metabolized to the 3 double bond-containing analog of 5-HETE viz., 5-HETrE (see Arachidonate 5-lipoxygenase § Mead acid).
Hydroxyeicosatetraenoic acids, leukotrienes, eoxins and prostanoids are sometimes termed "classic eicosanoids".[20][21][22]
Nonclassic eicosanoids
[edit]In contrast to the classic eicosanoids, several other classes of PUFA metabolites have been termed 'novel', 'eicosanoid-like' or 'nonclassic eicosanoids'.[23][24][25][26] These included the following classes:
- Oxoeicosanoids (oxo-ETE) include the following metabolites:
- 5-Oxo-eicosatetraenoic acid (5-oxo-ETE), 12-oxo-ETE (see 12-HETE § Further metabolism), and 15-oxo-ETE, which are metabolites of arachidonic acid (see 15-Hydroxyeicosatetraenoic acid) and 5-oxo-ETrE which is a metabolite of mead acid (see Arachidonate 5-lipoxygenase § Mead acid).
- Hepoxilins (Hx) include the following arachidonic acid metabolites:
- HxA3 and HxB3.
- Lipoxins (Lx) include the following metabolites of arachidonic acid:
- LxA4 and LxB4 (see Specialized pro-resolving mediators).
- Epi-lipoxins (epi-Lx) include the following metabolites of arachidonic acid:
- 15-epi-LxA4 (also termed AT-LxA4) and 15-epi-LxB4 (also termed AT-LxB4).
- Epoxyeicosatrienoic acids (EET) include the following metabolites of arachidonic acid:
- 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET.
- Epoxyeicosatetraenoic acids (EEQ) include the following metabolites of eicosapentaenoic acid:
- 5,6-EEQ, 8,9-EEQ, 11,12-EEQ, 14,15-EEQ, and 15,16-EEQ.
- Isoprostanes (isoP) are non-enzymatically formed derivatives of polyunsaturated fatty acids studied as markers of oxidative stress; they include the following arachidonic acid-derived isoPs which are named based on their structural similarities to PGs:[27][28]
- D2-isoPs, E2-isoPs, A2-isoPs, and J2-isoPs; and two epoxide-containing isoPs, 5,6-epoxyisoprostane E2 and 5,6-epoxyisoprostane A2. Some of these isoPs have been shown to possess anti-inflammatory activity (see Specialized pro-resolving mediators § Prostaglandins and isoprostanes).
- Isofurans are non-enzymatically formed derivatives of polyunsaturated fatty acids that possess a furan ring structure; they are studied as markers of oxidative stress. There are 256 potentially different furan ring-containing isomers that can be derived from arachidonic acid.[29]
- Endocannabinoids are certain glycerolipids or dopamine that are esterified to polyunsaturated fatty acids that activate cannabinoid receptors. They include the following arachidonic acid-esterified agents:
Metabolism of eicosapentaenoic acid to HEPEs, leukotrienes, prostanoids, and epoxyeicosatetraenoic acids as well as the metabolism of dihomo-gamma-linolenic acid to prostanoids and mead acid to 5(S)-hydroxy-6E,8Z,11Z-eicosatrienoic acid (5-HETrE), 5-oxo-6,8,11-eicosatrienoic acid (5-oxo-ETrE), LTA3, and LTC3 involve the same enzymatic pathways that make their arachidonic acid-derived analogs.
Biosynthesis
[edit]Eicosanoids typically are not stored within cells but rather synthesized as required. They derive from the fatty acids that make up the cell membrane and nuclear membrane. These fatty acids must be released from their membrane sites and then metabolized initially to products which most often are further metabolized through various pathways to make the large array of products we recognize as bioactive eicosanoids.
Fatty acid mobilization
[edit]Eicosanoid biosynthesis begins when a cell is activated by mechanical trauma, ischemia, other physical perturbations, attack by pathogens, or stimuli made by nearby cells, tissues, or pathogens such as chemotactic factors, cytokines, growth factors, and even certain eicosanoids. The activated cells then mobilize enzymes, termed phospholipases A2 (PLA2), capable of releasing ω−6 and ω−3 fatty acids from membrane storage. These fatty acids are bound in ester linkage to the SN2 position of membrane phospholipids; PLA2 act as esterases to release the fatty acid. There are several classes of PLA2 with type IV cytosolic PLA2 (cPLA2) appearing to be responsible for releasing the fatty acids under many conditions of cell activation. The cPLA2 act specifically on phospholipids that contain AA, EPA or GPLA at their SN2 position. cPLA2 may also release the lysophospholipid that becomes platelet-activating factor.[30]
Peroxidation and reactive oxygen species
[edit]Next, the free fatty acid is oxygenated along any of several pathways; see the Pathways table. The eicosanoid pathways (via lipoxygenase or COX) add molecular oxygen (O2). Although the fatty acid is symmetric, the resulting eicosanoids are chiral; the oxidations proceed with high stereoselectivity (enzymatic oxidations are considered practically stereospecific).
Four families of enzymes initiate or contribute to the initiation of the catalysis of fatty acids to eicosanoids:
- Cyclooxygenases (COXs): COX-1 and COX-2 initiate the metabolism of arachidonic acid to prostanoids that contain two double bonds, i.e. the prostaglandins (e.g. PGE2), prostacyclins (i.e. PGI2), and thromboxanes (e.g. TXA2). The two COX enzymes likewise initiate the metabolism of: a) Eicosapentaenoic acid, which has 5 double bonds compared to the 4 double bonds of arachidonic acid, to prostanoid, prostacyclin, and thromboxane products that have three double bonds, e.g. PGE3, PGI3, and TXA3 and b) Dihomo-γ-linolenic acid, which has three double bonds, to prostanoid, prostacyclin, and thromboxane products that have only one double bond, e.g. PGE1, PGI1, and TXA1.[31]
- Lipoxygenases (LOXs): 5-Lipoxygenase (5-LOX or ALOX5) initiates the metabolism of arachidonic acid to 5-hydroperoxyeicosatetraenoic acid (5-HpETE) which then may be rapidly reduced to 5-hydroxyeicosatetraenoic acid (5-HETE) or further metabolized to the leukotrienes (e.g. LTB4 and LTC4); 5-HETE may be oxidized to 5-oxo-eicosatetraenoic acid (5-oxo-ETE). In similar fashions, 15-lipoxygenase (15-lipoxygenase 1, 15-LOX, 15-LOX1, or ALOX15) initiates the metabolism of arachidonic acid to 15-HpETE, 15-HETE, eoxins, 8,15-dihydroxyeicosatetraenoic acid (i.e. 8,15-DiHETE), and 15-oxo-ETE and 12-lipoxygenase (12-LOX or ALOX12) initiates the metabolism of arachidonic acid to 12-HpETE, 12-HETE, hepoxilins, and 12-oxo-ETE. These enzymes also initiate the metabolism of; a) Eicosapentaenoic acid to analogs of the arachidonic acid metabolites that contain 5 rather than four double bonds, e.g. 5-hydroxyeicosapentaenoic acid (5-HEPE), LTB5, LTC5, 5-oxo-EPE, 15-HEPE, and 12-HEPE; b) The three double bond-containing dihomo-γ-linolenic acid to products that contain 3 double bonds, e.g. 8-hydroxy-eicosatrienoic acid (8-HETrE), 12-HETrE, and 15-HETrE (this fatty acid cannot be converted to leukotrienes); and the three double bond-containing mead acid (by ALOX5) to 5-hydroperoxy-eicosatrienoic acid (5-HpETrE), 5-HETrE, and 5-oxo-HETrE. In the most studied of these pathways, ALOX5 metabolizes eicosapentaenoic acid to 5-hydroperoxyeicosapentaenoic acid (5-HpEPE), 5-HEPE, and LTB5, and 5-oxo-EPE, all of which are less active than there arachidonic acid analogs. Since eicosapentaenoic acid competes with arachidonic acid for ALOX5, production of the eicosapentaenoate metabolites leads to a reduction in the eicosatetraenoate metabolites and therefore reduction in the latter metabolites' signaling.[31][32] The initial mono-hydroperoxy and mono-hydroxy products made by the aforementioned lipoxygenases have their hydroperosy and hydroxyl residues positioned in the S chiral configuration and are more properly termed 5S-HpETE, 5S-HETE, 12S-HpETE, 12S-HETE, 15S-HpETE and, 15S-HETE. ALOX12B (i.e. arachidonate 12-lipoxygenase, 12R type) forms R chirality products, i.e. 12R-HpETE and 12R-HETE. Similarly, ALOXE3 (i.e. epidermis-type lipoxygenase 3 or eLOX3) metabolizes arachidonic acid to 12R-HpETE and 12R-HETE; however these are minor products that this enzyme forms only under a limited set of conditions. ALOXE3 preferentially metabolizes arachidonic acid to hepoxilins.
- Epoxygenases: these are cytochrome P450 enzymes which generate nonclassic eicosanoid epoxides derived from: a) Arachidonic acid viz., 5,6-epoxy-eicosatrienoic acid (5,6-EET), 8,9-EET, 11,12-EET, and 14,15-EET (see Epoxyeicosatrienoic acid); b) Eicosapentaenoic acid viz., 5,6,-epoxy-eicosatetraenoic acid (5,6-EEQ), 8,9-EEQ, 11,12-EEQ, 14,15-EEQ, and 17,18-EEQ (see Epoxyeicosatetraenoic acid); c) Dihomo-γ-linolenic acid viz., 8,9-epoxy-eicosadienoic acid (8,9-EpEDE), 11,12-EpEDE, and 14,15-EpEDE; and d) Adrenic acid viz., 7,8-epox-eicosatrienoic acid (7,8-EpETrR), 10,11-EpTrE, 13,14-EpTrE, and 16,17-EpETrE. All of these epoxides are converted, sometimes rapidly, to their dihydroxy metabolites, by various cells and tissues. For example, 5,6-EET is converted to 5,6-dihydroxy-eicosatrienoic acid (5,6-DiHETrE), 8,9-EEQ to 8,9-dihydroxy-eicosatetraenoic acid (8,9-DiHETE, 11,12-EpEDE to 11,12-dihydroxy-eicosadienoic acid (11,12DiHEDE), and 16,17-EpETrE to 16,17-dihydroxy-eicosatrienoic acid (16,17-DiETrE.[31]
- Cytochrome P450 microsome ω hydroxylases: CYP4A11, CYP4A22, CYP4F2, and CYP4F3 metabolize arachidonic acid primarily to 20-hydroxyeicosatetraenoic acid (20-HETE) but also to 16-HETE, 17-HETE, 18-HETE, and 19-HETE; they also metabolize eicosapentaenoic acid primarily to 20-hydroxy-eicosapentaenoic acid (20-HEPE) but also to 19-HEPE.[31]
Two different enzymes may act in series on a PUFA to form more complex metabolites. For example, ALOX5 acts with ALOX12 or aspirin-treated COX-2 to metabolize arachidonic acid to lipoxins and with cytochrome P450 monooxygenase(s), bacterial cytochrome P450 (in infected tissues), or aspirin-treated COX2 to metabolize eicosapentaenoic acid to the E series resolvins (RvEs) (see Specialized pro-resolving mediators). When this occurs with enzymes located in different cell types and involves the transfer of one enzyme's product to a cell which uses the second enzyme to make the final product it is referred to as transcellular metabolism or transcellular biosynthesis.[33]
The oxidation of lipids is hazardous to cells, particularly when close to the nucleus. There are elaborate mechanisms to prevent unwanted oxidation. COX, the lipoxygenases, and the phospholipases are tightly controlled—there are at least eight proteins activated to coordinate generation of leukotrienes. Several of these exist in multiple isoforms.[7]
Oxidation by either COX or lipoxygenase releases reactive oxygen species (ROS) and the initial products in eicosanoid generation are themselves highly reactive peroxides. LTA4 can form adducts with tissue DNA. Other reactions of lipoxygenases generate cellular damage; murine models implicate 15-lipoxygenase in the pathogenesis of atherosclerosis.[34][35] The oxidation in eicosanoid generation is compartmentalized; this limits the peroxides' damage. The enzymes that are biosynthetic for eicosanoids (e.g., glutathione-S-transferases, epoxide hydrolases, and carrier proteins) belong to families whose functions are involved largely with cellular detoxification. This suggests that eicosanoid signaling might have evolved from the detoxification of ROS.
The cell must realize some benefit from generating lipid hydroperoxides close-by its nucleus. PGs and LTs may signal or regulate DNA transcription there; LTB4 is ligand for PPARα.[5] (See diagram at PPAR.)
Prostanoid pathways
[edit]Both COX1 and COX2 (also termed prostaglandin-endoperoxide synthase-1 (PTGS1) and PTGS2, respectively) metabolize arachidonic acid by adding molecular O2 between carbons 9 and 11 to form an endoperoxide bridge between these two carbons, adding molecular O2 to carbon 15 to yield a 15-hydroperoxy product, creating a carbon-carbon bond between carbons 8 and 12 to create a cyclopentane ring in the middle of the fatty acid, and in the process making PGG2, a product that has two fewer double bonds than arachidonic acid. The 15-hydroperoxy residue of PGG2 is then reduced to a 15-hydroxyl residue thereby forming PGH2. PGH2 is the parent prostanoid to all other prostanoids. It is metabolized by (see diagram in Prostanoid): a) The prostaglandin E synthase pathway in which any one of three isozymes, PTGES, PTGES2, or PTGES3, convert PGH2 to PGE2 (subsequent products of this pathway include PGA2 and PGB2 (see Prostanoid § Biosynthesis of prostaglandins); b) PGF synthase which converts PGH2 to PGF2α; c) Prostaglandin D2 synthase which converts PGH2 to PGD2 (subsequent products in this pathway include 15-dPGJ2 (see Cyclopentenone prostaglandin); d) Thromboxane synthase which converts PGH2 to TXA2 (subsequent products in this pathway include TXB2); and e) Prostacyclin synthase which converts PGH2 to PGI2 (subsequent products in this pathway include 6-keto-PGFα.[36][37] These pathways have been shown or in some cases presumed to metabolize eicosapentaenoic acid to eicosanoid analogs of the sited products that have three rather than two double bonds and therefore contain the number 3 in place of 2 attached to their names (e.g. PGE3 instead of PGE2).[38]
The PGE2, PGE1, and PGD2 products formed in the pathways just cited can undergo a spontaneous dehydration reaction to form PGA2, PGA1, and PGJ2, respectively; PGJ2 may then undergo a spontaneous isomerization followed by a dehydration reaction to form in series Δ12-PGJ2 and 15-deoxy-Δ12,14-PGJ2.[39]
PGH2 has a 5-carbon ring bridged by molecular oxygen. Its derived PGS have lost this oxygen bridge and contain a single, unsaturated 5-carbon ring with the exception of thromboxane A2 which possesses a 6-member ring consisting of one oxygen and 5 carbon atoms. The 5-carbon ring of prostacyclin is conjoined to a second ring consisting of 4 carbon and one oxygen atom. And, the 5 member ring of the cyclopentenone prostaglandins possesses an unsaturated bond in a conjugated system with a carbonyl group that causes these PGs to form bonds with a diverse range of bioactive proteins (for more see the diagrams at Prostanoid).
Hydroxyeicosatetraenoate (HETE) and leukotriene (LT) pathways
[edit]The enzyme 5-lipoxygenase (5-LO or ALOX5) converts arachidonic acid into 5-hydroperoxyeicosatetraenoic acid (5-HPETE), which may be released and rapidly reduced to 5-hydroxyeicosatetraenoic acid (5-HETE) by ubiquitous cellular glutathione-dependent peroxidases.[40] Alternately, ALOX5 uses its LTA synthase activity to act convert 5-HPETE to leukotriene A4 (LTA4). LTA4 is then metabolized either to LTB4 by leukotriene A4 hydrolase or leukotriene C4 (LTC4) by either LTC4 synthase or microsomal glutathione S-transferase 2 (MGST2). Either of the latter two enzymes act to attach the sulfur of cysteine's thio- (i.e. SH) group in the tripeptide glutamate-cysteine-glycine to carbon 6 of LTA4 thereby forming LTC4. After release from its parent cell, the glutamate and glycine residues of LTC4 are removed step-wise by gamma-glutamyltransferase and a dipeptidase to form sequentially LTD4 and LTE4.[41][42] The decision to form LTB4 versus LTC4 depends on the relative content of LTA4 hydrolase versus LTC4 synthase (or glutathione S-transferase in cells; eosinophils, mast cells, and alveolar macrophages possess relatively high levels of LTC4 synthase and accordingly form LTC4 rather than or to a far greater extent than LTB4. 5-LOX may also work in series with cytochrome P450 oxygenases or aspirin-treated COX2 to form Resolvins RvE1, RvE2, and 18S-RvE1 (see Specialized pro-resolving mediators § EPA-derived resolvins).
The enzyme arachidonate 12-lipoxygenase (12-LO or ALOX12) metabolizes arachidonic acid to the S stereoisomer of 12-hydroperoxyeicosatetraenoic acid (12-HPETE) which is rapidly reduced by cellular peroxidases to the S stereoisomer of 12-hydroxyeicosatetraenoic acid (12-HETE) or further metabolized to hepoxilins (Hx) such as HxA3 and HxB.[43][44]
The enzymes 15-lipoxygenase-1 (15-LO-1 or ALOX15) and 15-lipoxygenase-2 (15-LO-2, ALOX15B) metabolize arachidonic acid to the S stereoisomer of 15-hydroperoxyeicosatetraenoic acid (15(S)-HPETE) which is rapidly reduced by cellular peroxidases to the S stereoisomer of 15-hydroxyeicosatetraenoic acid (15(S)-HETE).[45][46] The 15-lipoxygenases (particularly ALOX15) may also act in series with 5-lipoxygenase, 12-lipoxygenase, or aspirin-treated COX2 to form the lipoxins and epi-lipoxins or with P450 oxygenases or aspirin-treated COX2 to form Resolvin E3 (see Specialized pro-resolving mediators § EPA-derived resolvins).
A subset of cytochrome P450 (CYP450) microsome-bound ω hydroxylases metabolize arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE) and 19-hydroxyeicosatetraenoic acid by an omega oxidation reaction.[47]
Epoxyeicosanoid pathway
[edit]The human cytochrome P450 (CYP) epoxygenases, CYP1A1, CYP1A2, CYP2C8, CYP2C9, CYP2C18, CYP2C19, CYP2E1, CYP2J2, and CYP2S1 metabolize arachidonic acid to the non-classic epoxyeicosatrienoic acids (EETs) by converting one of the fatty acid's double bonds to its epoxide to form one or more of the following EETs, 14,15-ETE, 11,12-EET, 8,9-ETE, and 4,5-ETE.[48][49] 14,15-EET and 11,12-EET are the major EETs produced by mammalian, including human, tissues.[49][50][51][52][53] The same CYPs but also CYP4A1, CYP4F8, and CYP4F12 metabolize eicosapentaenoic acid to five epoxide epoxyeicosatetraenoic acids (EEQs) viz., 17,18-EEQ, 14,15-EEQ, 11,12-EEQ. 8,9-EEQ, and 5,6-EEQ.[54]
Function, pharmacology, and clinical significance
[edit]The following table lists a sampling of the major eicosanoids that possess clinically relevant biological activity, the cellular receptors (see Cell surface receptor) that they stimulate or, where noted, antagonize to attain this activity, some of the major functions which they regulate (either promote or inhibit) in humans and mouse models, and some of their relevancies to human diseases.
| Eicosanoid | Targeted receptors | Functions regulated | Clinical relevancy |
|---|---|---|---|
| PGE2 | PTGER1, PTGER2, PTGER3, PTGER4 | inflammation; fever; pain perception; allodynia; parturition | NSAIDs inhibit its production to reduce inflammation, fever, and pain; used to promote labor in childbirth; an abortifacient[37][55][56] |
| PGD2 | Prostaglandin DP1 receptor 1, Prostaglandin DP2 receptor | allergy reactions; allodynia; hair growth | NSAIDs may target it to inhibit allodynia and male-pattern hair loss[37][57][58][59][60] |
| TXA2 | Thromboxane receptor α and β | blood platelet aggregation; blood clotting; allergic reactions | NSAIDs inhibit its production to reduce incidence of strokes and heart attacks[37][61] |
| PGI2 | Prostacyclin receptor | platelet aggregation, vascular smooth muscle contraction | PGI2 analogs used to treat vascular disorders like pulmonary hypertension, Raynaud's syndrome, and Buerger's disease[62][63][64] |
| 15-d-Δ12,14-PGJ2 | PPARγ, Prostaglandin DP2 receptor | inhibits inflammation and cell growth | inhibits diverse inflammatory responses in animal models; structural model for developing anti-inflammatory agents[12][59][60] |
| 20-HETE | ? | vasoconstriction, inhibits platelets | inactivating mutations in the 20-HETE-forming enzyme, CYP2U1, associated with hereditary spastic paraplegia[65] |
| 5-Oxo-ETE | OXER1 | chemotactic factor for and activator of eosinophils | studies needed to determine if inhibiting its production or action inhibits allergic reactons[32] |
| LTB4 | LTB4R, LTB4R2 | chemotactic factor for and activator of leukocytes; inflammation | studies to date shown no clear benefits of LTB4 receptor antagonists for human inflammatory diseases[66][67][68] |
| LTC4 | CYSLTR1, CYSLTR2, GPR17 | vascular permeability; vascular smooth muscle contraction; allergy | antagonists of CYSLTR1 used in asthma as well as other allergic and allergic-like reactions[69][70] |
| LTD4 | CYSLTR1, CYSLTR2, GPR17 | vascular permeability; vascular smooth muscle contraction; allergy | antagonists of CYSLTR1 used in asthma as well as other allergic and allergic-like reactions[66] |
| LTE4 | GPR99 | increases vascular permeability and airway mucin secretion | thought to contribute to asthma as well as other allergic and allergic-like reactions[71] |
| LxA4 | FPR2 | inhibits functions of pro-inflammatory cells | Specialized pro-resolving mediators class of inflammatory reaction suppressors[72][73] |
| LxB4 | FPR2, GPR32, AHR | inhibits functions of pro-inflammatory cells | Specialized pro-resolving mediators class of inflammatory reaction suppressors[72][73] |
| RvE1 | CMKLR1, inhibits BLT, TRPV1, TRPV3, NMDAR, TNFR | inhibits functions of pro-inflammatory cells | Specialized pro-resolving mediators class of inflammatory reaction suppressors; also suppresses pain perception[74][75][76] |
| RvE2 | CMKLR1, receptor antagonist of BLT | inhibits functions of pro-inflammatory cells | Specialized pro-resolving mediators class of inflammatory reaction suppressors[72][73][76][77] |
| 14,15-EET | ? | vasodilation, inhibits platelets and pro-inflammatory cells | role(s) in human disease not yet proven[78][79] |
Prostanoids
[edit]Many of the prostanoids are known to mediate local symptoms of inflammation: vasoconstriction or vasodilation, coagulation, pain, and fever. Inhibition of COX-1 and/or the inducible COX-2 isoforms is the hallmark of NSAIDs (non-steroidal anti-inflammatory drugs), such as aspirin. Prostanoids also activate the PPARγ members of the steroid/thyroid family of nuclear hormone receptors, and directly influence gene transcription.[80] Prostanoids have numerous other relevancies to clinical medicine as evidence by their use, the use of their more stable pharmacological analogs, of the use of their receptor antagonists as indicated in the following chart.
| Medicine | Type | Medical condition or use | Medicine | Type | Medical condition or use | |
|---|---|---|---|---|---|---|
| Alprostadil | PGE1 | Erectile dysfunction, maintaining a patent ductus arteriosus in the fetus | Beraprost | PGI2 analog | Pulmonary hypertension, avoiding reperfusion injury | |
| Bimatoprost | PGF2α analog | Glaucoma, ocular hypertension | Carboprost | PGF2α analog | Labor induction, abortifacient in early pregnancy | |
| Dinoprostone | PGE2 | Labor induction | Iloprost | PGI2 analog | Pulmonary artery hypertension | |
| Latanoprost | PGF2α analog | Glaucoma, ocular hypertension | Misoprostol | PGE1 analog | Stomach ulcers labor induction, abortifacient | |
| Travoprost | PGF2α analog | Glaucoma, ocular hypertension | U46619 | Longer lived TX analog | Research only |
Cyclopentenone prostaglandins
[edit]PGA1, PGA2, PGJ2, Δ12-PGJ2, and 15-deox-Δ12,14-PGJ2 exhibit a wide range of anti-inflammatory and inflammation-resolving actions in diverse animal models.[39] They therefore appear to function in a manner similar to specialized pro-resolving mediators although one of their mechanisms of action, forming covalent bonds with key signaling proteins, differs from those of the specialized pro-resolving mediators.
HETEs and oxo-ETEs
[edit]As indicated in their individual Wikipedia pages, 5-hydroxyeicosatetraenoic acid (which, like 5-oxo-eicosatetraenoic acid, acts through the OXER1 receptor), 5-oxo-eicosatetraenoic acid, 12-hydroxyeicosatetraenoic acid, 15-hydroxyeicosatetraenoic acid, and 20-hydroxyeicosatetraenoic acid show numerous activities in animal and human cells as well as in animal models that are related to, for example, inflammation, allergic reactions, cancer cell growth, blood flow to tissues, and/or blood pressure. However, their function and relevancy to human physiology and pathology have not as yet been shown.
Leukotrienes
[edit]The three cysteinyl leukotrienes, LTC4, LTD4, and LTE4, are potent bronchoconstrictors, increasers of vascular permeability in postcapillary venules, and stimulators of mucus secretion that are released from the lung tissue of asthmatic subjects exposed to specific allergens. They play a pathophysiological role in diverse types of immediate hypersensitivity reactions.[81] Drugs that block their activation of the CYSLTR1 receptor viz., montelukast, zafirlukast, and pranlukast, are used clinically as maintenance treatment for allergen-induced asthma and rhinitis; nonsteroidal anti-inflammatory drug-induced asthma and rhinitis (see aspirin-exacerbated respiratory disease); exercise- and cold-air induced asthma (see Exercise-induced bronchoconstriction); and childhood sleep apnea due to adenotonsillar hypertrophy (see Acquired non-inflammatory myopathy § Diet and Trauma Induced Myopathy).[82][83][84][85] When combined with antihistamine drug therapy, they also appear useful for treating urticarial diseases such as hives.[86]
Lipoxins and epi-lipoxins
[edit]LxA4, LxB4, 15-epi-LxA4, and 15-epi-LXB4, like other members of the specialized pro-resolving mediators class of eicosanoids, possess anti-inflammatory and inflammation resolving activity. In a randomized controlled trial, AT-LXA4 and a comparatively stable analog of LXB4, 15R/S-methyl-LXB4, reduced the severity of eczema in a study of 60 infants[87] and, in another study, inhaled LXA4 decreased LTC4-initiated bronchoprovocation in patients with asthma.[88]
Eoxins
[edit]The eoxins (EXC4, EXD4, EXE5) are newly described. They stimulate vascular permeability in an ex vivo human vascular endothelial model system,[89] and in a small study of 32 volunteers EXC4 production by eosinophils isolated from severe and aspirin-intolerant asthmatics was greater than that from healthy volunteers and mild asthmatic patients; these findings have been suggested to indicate that the eoxins have pro-inflammatory actions and therefore potentially involved in various allergic reactions.[90] Production of eoxins by Reed–Sternberg cells cells has also led to suggestion that they are involved in Hodgkins disease.[91] However, the clinical significance of eoxins has not yet been demonstrated.
Resolvin metabolites of eicosapentaenoic acid
[edit]RvE1, 18S-RvE1, RvE2, and RvE3, like other members of the specialized pro-resolving mediators) class of eicosanoids, possess anti-inflammatory and inflammation resolving activity. A synthetic analog of RvE1 is in clinical phase III testing (see Phases of clinical research) for the treatment of the inflammation-based dry eye syndrome; along with this study, other clinical trials (NCT01639846, NCT01675570, NCT00799552 and NCT02329743) using an RvE1 analogue to treat various ocular conditions are underway.[88] RvE1 is also in clinical development studies for the treatment of neurodegenerative diseases and hearing loss.[92]
Other metabolites of eicosapentaenoic acid
[edit]The metabolites of eicosapentaenoic acid that are analogs of their arachidonic acid-derived prostanoid, HETE, and LT counterparts include: the 3-series prostanoids (e.g. PGE3, PGD3, PGF3α, PGI3, and TXA3), the hydroxyeicosapentaenoic acids (e.g. 5-HEPE, 12-HEPE, 15-HEPE, and 20-HEPE), and the 5-series LTs (e.g. LTB5, LTC5, LTD5, and LTE5). Many of the 3-series prostanoids, the hydroxyeicosapentaenoic acids, and the 5-series LT have been shown or thought to be weaker stimulators of their target cells and tissues than their arachidonic acid-derived analogs. They are proposed to reduce the actions of their arachidonate-derived analogs by replacing their production with weaker analogs.[93][94] Eicosapentaenoic acid-derived counterparts of the eoxins have not been described.
Epoxyeicosanoids
[edit]The epoxy eicosatrienoic acids (or EETs)—and, presumably, the epoxy eicosatetraenoic acids—have vasodilating actions on heart, kidney, and other blood vessels as well as on the kidney's reabsorption of sodium and water, and act to reduce blood pressure and ischemic and other injuries to the heart, brain, and other tissues; they may also act to reduce inflammation, promote the growth and metastasis of certain tumors, promote the growth of new blood vessels, in the central nervous system, regulate the release of neuropeptide hormones, and in the peripheral nervous system inhibit or reduce pain perception.[48][49][51]
The ω−3 and ω−6 series
[edit]The reduction in AA-derived eicosanoids and the diminished activity of the alternative products generated from ω-3 fatty acids serve as the foundation for explaining some of the beneficial effects of greater ω-3 intake.
— Kevin Fritsche, Fatty Acids as Modulators of the Immune Response[95]
Arachidonic acid (AA; 20:4 ω−6) sits at the head of the "arachidonic acid cascade" – more than twenty eicosanoid-mediated signaling paths controlling a wide array of cellular functions, especially those regulating inflammation, immunity, and the central nervous system.[6]
In the inflammatory response, two other groups of dietary fatty acids form cascades that parallel and compete with the arachidonic acid cascade. EPA (20:5 ω−3) provides the most important competing cascade. DGLA (20:3 ω−6) provides a third, less prominent cascade. These two parallel cascades soften the inflammatory effects of AA and its products. Low dietary intake of these less-inflammatory fatty acids, especially the ω−3s, has been linked to several inflammation-related diseases, and perhaps some mental illnesses.
The U.S. National Institutes of Health and the National Library of Medicine state that there is 'A' level evidence that increased dietary ω−3 improves outcomes in hypertriglyceridemia, secondary cardiovascular disease prevention, and hypertension. There is 'B' level evidence ('good scientific evidence') for increased dietary ω−3 in primary prevention of cardiovascular disease, rheumatoid arthritis, and protection from ciclosporin toxicity in organ transplant patients. They also note more preliminary evidence showing that dietary ω−3 can ease symptoms in several psychiatric disorders.[96]
Besides the influence on eicosanoids, dietary polyunsaturated fats modulate immune response through three other molecular mechanisms. They (a) alter membrane composition and function, including the composition of lipid rafts; (b) change cytokine biosynthesis; and (c) directly activate gene transcription.[95] Of these, the action on eicosanoids is the best explored
Recent data in 2024 has emerged that neuronal integrity breakdown was reduced by ω−3 treatment in APOE*E4 carriers, suggesting that this treatment may be beneficial for this specific group suggested fish oil supplements might help older adults fight Alzheimer’s disease.[97][98]
Mechanisms of ω−3 action
[edit]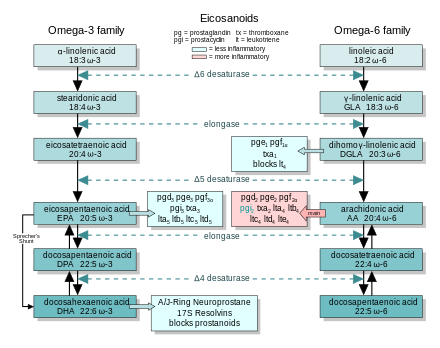
In general, the eicosanoids derived from AA promote inflammation, and those from EPA and from GLA (via DGLA) are less inflammatory, or inactive, or even anti-inflammatory and pro-resolving.
The figure shows the ω−3 and −6 synthesis chains, along with the major eicosanoids from AA, EPA, and DGLA.
Dietary ω−3 and GLA counter the inflammatory effects of AA's eicosanoids in three ways, along the eicosanoid pathways:
- Displacement—Dietary ω−3 decreases tissue concentrations of AA, so there is less to form ω−6 eicosanoids.
- Competitive inhibition—DGLA and EPA compete with AA for access to the cyclooxygenase and lipoxygenase enzymes. So the presence of DGLA and EPA in tissues lowers the output of AA's eicosanoids.
- Counteraction—Some DGLA and EPA derived eicosanoids counteract their AA derived counterparts.
Role in inflammation
[edit]Since antiquity, the cardinal signs of inflammation have been known as: calor (warmth), dolor (pain), tumor (swelling), and rubor (redness). The eicosanoids are involved with each of these signs.
Redness—An insect's sting will trigger the classic inflammatory response. Short acting vasoconstrictors — TXA2 — are released quickly after the injury. The site may momentarily turn pale. Then TXA2 mediates the release of the vasodilators PGE2 and LTB4. The blood vessels engorge and the injury reddens.
Swelling—LTB4 makes the blood vessels more permeable. Plasma leaks out into the connective tissues, and they swell. The process also loses pro-inflammatory cytokines.
Pain—The cytokines increase COX-2 activity. This elevates levels of PGE2, sensitizing pain neurons.
Heat—PGE2 is also a potent pyretic agent. Aspirin and NSAIDS—drugs that block the COX pathways and stop prostanoid synthesis—limit fever or the heat of localized inflammation.
History
[edit]In 1930, gynecologist Raphael Kurzrok and pharmacologist Charles Leib characterized prostaglandin as a component of semen. Between 1929 and 1932, George and Mildred Burr showed that restricting fat from animals' diets led to a deficiency disease, and first described the essential fatty acids.[99] In 1935, von Euler identified prostaglandin. In 1964, Bergström and Samuelsson linked these observations when they showed that the "classical" eicosanoids were derived from arachidonic acid, which had earlier been considered to be one of the essential fatty acids.[100] In 1971, Vane showed that aspirin and similar drugs inhibit prostaglandin synthesis.[101] Von Euler received the Nobel Prize in medicine in 1970, which Samuelsson, Vane, and Bergström also received in 1982. E. J. Corey received it in chemistry in 1990 largely for his synthesis of prostaglandins.
See also
[edit]References
[edit]- ^ a b "Eicosanoid Synthesis and Metabolism: Prostaglandins, Thromboxanes, Leukotrienes, Lipoxins". The Medical Biochemistry Page. 2024. Retrieved 9 April 2024.
- ^ "15.2C: Chemistry of Hormones". Medicine LibreTexts. 2018-07-21. Retrieved 2024-04-09.
- ^ Edwards IJ, O'Flaherty JT (2008). "Omega-3 Fatty Acids and PPARgamma in Cancer". PPAR Research. 2008: 358052. doi:10.1155/2008/358052. PMC 2526161. PMID 18769551.
- ^ DeCaterina, R; Basta, G (June 2001). "n-3 Fatty acids and the inflammatory response – biological background". European Heart Journal Supplements. 3, Suppl D: D42 – D49. doi:10.1016/S1520-765X(01)90118-X. S2CID 22691568.
- ^ a b Funk, Colin D. (30 November 2001). "Prostaglandins and Leukotrienes: Advances in Eicosanoid Biology". Science. 294 (5548): 1871–1875. Bibcode:2001Sci...294.1871F. doi:10.1126/science.294.5548.1871. PMID 11729303.
- ^ a b Piomelli, Daniele (2000). "Arachidonic Acid". Neuropsychopharmacology: The Fifth Generation of Progress. Archived from the original on 2006-07-15. Retrieved 2006-03-03.
- ^ a b Soberman, Roy J.; Christmas, Peter (2003). "The organization and consequences of eicosanoid signaling". J. Clin. Invest. 111 (8): 1107–1113. doi:10.1172/JCI18338. PMC 152944. PMID 12697726.
- ^ Beare-Rogers (2001). "IUPAC Lexicon of Lipid Nutrition" (PDF). Retrieved June 1, 2006.
- ^ Prostacyclin—PGI—was previously classified as prostaglandin and retains its old PGI2 identifier.
- ^ Eicosanoids with different letters have placement of double-bonds and different functional groups attached to the molecular skeleton. Letters indicate roughly the order the eicosanoids were first described in the literature. For diagrams for PG [A–H] see Cyberlipid Center. "Prostanoids". Archived from the original on 2007-02-08. Retrieved 2007-02-05.
- ^ Rossi AG, Thomas MJ, O'Flaherty JT (1988). "Stereospecific actions of 5-hydroxyeicosatetraenoate". FEBS Letters. 240 (1–2): 163–166. doi:10.1016/0014-5793(88)80360-0. PMID 3191990. S2CID 43027447.
- ^ a b Straus DS, Glass CK (2001). "Cyclopentenone prostaglandins: new insights on biological activities and cellular targets". Medicinal Research Reviews. 21 (3): 185–210. doi:10.1002/med.1006.abs. PMID 11301410.
- ^ Prasad KN, Hovland AR, Cole WC, Prasad KC, Nahreini P, Edwards-Prasad J, Andreatta CP (2000). "Multiple antioxidants in the prevention and treatment of Alzheimer disease: analysis of biologic rationale". Clinical Neuropharmacology. 23 (1): 2–13. doi:10.1097/00002826-200001000-00002. PMID 10682224.
- ^ Xu Y, Qian SY (2014). "Anti-cancer activities of ω-6 polyunsaturated fatty acids". Biomedical Journal. 37 (3): 112–119. doi:10.4103/2319-4170.131378. PMC 4166599. PMID 24923568.
- ^ Gomolka B, Siegert E, Blossey K, Schunck WH, Rothe M, Weylandt KH (2011). "Analysis of omega-3 and omega-6 fatty acid-derived lipid metabolite formation in human and mouse blood samples". Prostaglandins & Other Lipid Mediators. 94 (3–4): 81–87. doi:10.1016/j.prostaglandins.2010.12.006. PMID 21236358.
- ^ Zulfakar MH, Edwards M, Heard CM (2007). "Is there a role for topically delivered eicosapentaenoic acid in the treatment of psoriasis?". European Journal of Dermatology. 17 (4): 284–291. doi:10.1684/ejd.2007.0201 (inactive 1 November 2024). PMID 17540633.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Caramia G (2012). "[Essential fatty acids and lipid mediators. Endocannabinoids]". La Pediatria Medica e Chirurgica: Medical and Surgical Pediatrics (in Italian). 34 (2): 65–72. doi:10.4081/pmc.2012.2. PMID 22730630.
- ^ a b c d Wiktorowska-Owczarek A, Berezińska M, Nowak JZ (2015). "PUFAs: Structures, Metabolism and Functions". Advances in Clinical and Experimental Medicine. 24 (6): 931–941. doi:10.17219/acem/31243. PMID 26771963.
- ^ Tanaka N, Yamaguchi H, Furugen A, Ogura J, Kobayashi M, Yamada T, Mano N, Iseki K (2014). "Quantification of intracellular and extracellular eicosapentaenoic acid-derived 3-series prostanoids by liquid chromatography/electrospray ionization tandem mass spectrometry". Prostaglandins, Leukotrienes, and Essential Fatty Acids. 91 (3): 61–71. doi:10.1016/j.plefa.2014.04.005. PMID 24996760.
- ^ Van Dyke TE, Serhan CN (2003). "Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases". J. Dent. Res. 82 (2): 82–90. doi:10.1177/154405910308200202. PMID 12562878. S2CID 40812937.
- ^ Serhan CN, Gotlinger K, Hong S, Arita M (2004). "Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis". Prostaglandins Other Lipid Mediat. 73 (3–4): 155–172. doi:10.1016/j.prostaglandins.2004.03.005. PMID 15290791.
- ^ Anderle P, Farmer P, Berger A, Roberts MA (2004). "Nutrigenomic approach to understanding the mechanisms by which dietary long-chain fatty acids induce gene signals and control mechanisms involved in carcinogenesis". Nutrition (Burbank, Los Angeles County, Calif.). 20 (1): 103–108. doi:10.1016/j.nut.2003.09.018. PMID 14698023.
- ^ Evans AR, Junger H, Southall MD, et al. (2000). "Isoprostanes, novel eicosanoids that produce nociception and sensitize rat sensory neurons". J. Pharmacol. Exp. Ther. 293 (3): 912–920. PMID 10869392.
- ^ O'Brien WF, Krammer J, O'Leary TD, Mastrogiannis DS (1993). "The effect of acetaminophen on prostacyclin production in pregnant women". Am. J. Obstet. Gynecol. 168 (4): 1164–1169. doi:10.1016/0002-9378(93)90362-m. PMID 8475962.
- ^ Behrendt H, Kasche A, Ebner von Eschenbach C, Risse U, Huss-Marp J, Ring J (2001). "Secretion of proinflammatory eicosanoid-like substances precedes allergen release from pollen grains in the initiation of allergic sensitization" (PDF). Int. Arch. Allergy Immunol. 124 (1–3): 121–125. doi:10.1159/000053688. PMID 11306946. S2CID 53331.
- ^ Sarau HM, Foley JJ, Schmidt DB, et al. (1999). "In vitro and in vivo pharmacological characterization of SB 201993, an eicosanoid-like LTB4 receptor antagonist with anti-inflammatory activity". Prostaglandins Leukot. Essent. Fatty Acids. 61 (1): 55–64. doi:10.1054/plef.1999.0074. PMID 10477044.
- ^ Czerska M, Zieliński M, Gromadzińska J (2016). "Isoprostanes - A novel major group of oxidative stress markers". International Journal of Occupational Medicine and Environmental Health. 29 (2): 179–190. doi:10.13075/ijomeh.1896.00596. PMID 26670350.
- ^ Friedli O, Freigang S (2016). "Cyclopentenone-containing oxidized phospholipids and their isoprostanes as pro-resolving mediators of inflammation". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1862 (4): 382–392. doi:10.1016/j.bbalip.2016.07.006. PMID 27422370.
- ^ Cuyamendous C, de la Torre A, Lee YY, Leung KS, Guy A, Bultel-Poncé V, Galano JM, Lee JC, Oger C, Durand T (2016). "The novelty of phytofurans, isofurans, dihomo-isofurans and neurofurans: Discovery, synthesis and potential application" (PDF). Biochimie. 130: 49–62. doi:10.1016/j.biochi.2016.08.002. PMID 27519299. S2CID 1504539.
- ^ University of Kansas Medical Center (2004). "Eicosanoids and Inflammation" (PDF). Archived from the original (PDF) on 2005-05-16. Retrieved 2007-01-05.
- ^ a b c d Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM (2015). "Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs". Advances in Nutrition. 6 (5): 513–540. doi:10.3945/an.114.007732. PMC 4561827. PMID 26374175.
- ^ a b Powell WS, Rokach J (2015). "Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1851 (4): 340–355. doi:10.1016/j.bbalip.2014.10.008. PMC 5710736. PMID 25449650.
- ^ Capra V, Rovati GE, Mangano P, Buccellati C, Murphy RC, Sala A (2015). "Transcellular biosynthesis of eicosanoid lipid mediators". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1851 (4): 377–382. doi:10.1016/j.bbalip.2014.09.002. PMID 25218301.
- ^ Cyrus, Tillmann; Witztum, Joseph L.; Rader, Daniel J.; Tangirala, Rajendra; Fazio, Sergio; Linton, Macrae F.; Funk, Colin D. (June 1999). "Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E–deficient mice". J Clin Invest. 103 (11): 1597–1604n. doi:10.1172/JCI5897. PMC 408369. PMID 10359569.
- ^ Schewe T. (Mar–Apr 2002). "15-lipoxygenase-1: a prooxidant enzyme". Biol. Chem. 383 (3–4): 365–374. doi:10.1515/BC.2002.041. PMID 12033428. S2CID 7487557.
- ^ Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D (2014). "Cyclooxygenase pathways". Acta Biochimica Polonica. 61 (4): 639–649. doi:10.18388/abp.2014_1825. PMID 25343148.
- ^ a b c d Claar D, Hartert TV, Peebles RS (2015). "The role of prostaglandins in allergic lung inflammation and asthma". Expert Review of Respiratory Medicine. 9 (1): 55–72. doi:10.1586/17476348.2015.992783. PMC 4380345. PMID 25541289.
- ^ Simopoulos AP (2010). "Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk". Experimental Biology and Medicine. 235 (7): 785–795. doi:10.1258/ebm.2010.009298. PMID 20558833. S2CID 207195131.
- ^ a b Surh YJ, Na HK, Park JM, Lee HN, Kim W, Yoon IS, Kim DD (2011). "15-Deoxy-Δ¹²,¹⁴-prostaglandin J₂, an electrophilic lipid mediator of anti-inflammatory and pro-resolving signaling". Biochemical Pharmacology. 82 (10): 1335–1351. doi:10.1016/j.bcp.2011.07.100. PMID 21843512.
- ^ Powell, W. S.; Rokach, J (2013). "The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor". Progress in Lipid Research. 52 (4): 651–665. doi:10.1016/j.plipres.2013.09.001. PMC 5710732. PMID 24056189.
- ^ Rådmark O, Werz O, Steinhilber D, Samuelsson B (2015). "5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1851 (4): 331–339. doi:10.1016/j.bbalip.2014.08.012. PMID 25152163.
- ^ Ahmad S, Thulasingam M, Palombo I, Daley DO, Johnson KA, Morgenstern R, Haeggström JZ, Rinaldo-Matthis A (2015). "Trimeric microsomal glutathione transferase 2 displays one third of the sites reactivity". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1854 (10 Pt A): 1365–1371. doi:10.1016/j.bbapap.2015.06.003. PMID 26066610.
- ^ Pace-Asciak, C. R. (2009). "The hepoxilins and some analogues: A review of their biology". British Journal of Pharmacology. 158 (4): 972–981. doi:10.1111/j.1476-5381.2009.00168.x. PMC 2785520. PMID 19422397.
- ^ Dobrian, A. D.; Lieb, D. C.; Cole, B. K.; Taylor-Fishwick, D. A.; Chakrabarti, S. K.; Nadler, J. L. (2011). "Functional and pathological roles of the 12- and 15-lipoxygenases". Progress in Lipid Research. 50 (1): 115–131. doi:10.1016/j.plipres.2010.10.005. PMC 3012140. PMID 20970452.
- ^ Ivanov, I; Kuhn, H; Heydeck, D (2015). "Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15)". Gene. 573 (1): 1–32. doi:10.1016/j.gene.2015.07.073. PMC 6728142. PMID 26216303.
- ^ Wittwer, J; Hersberger, M (2007). "The two faces of the 15-lipoxygenase in atherosclerosis". Prostaglandins, Leukotrienes and Essential Fatty Acids. 77 (2): 67–77. doi:10.1016/j.plefa.2007.08.001. PMID 17869078.
- ^ Kroetz DL, Xu F (2005). "Regulation and inhibition of arachidonic acid omega-hydroxylases and 20-HETE formation". Annual Review of Pharmacology and Toxicology. 45: 413–438. doi:10.1146/annurev.pharmtox.45.120403.100045. PMID 15822183.
- ^ a b Yang, L; Mäki-Petäjä, K; Cheriyan, J; McEniery, C; Wilkinson, I. B. (2015). "The role of epoxyeicosatrienoic acids in the cardiovascular system". British Journal of Clinical Pharmacology. 80 (1): 28–44. doi:10.1111/bcp.12603. PMC 4500322. PMID 25655310.
- ^ a b c Spector, A. A.; Kim, H. Y. (2015). "Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1851 (4): 356–365. doi:10.1016/j.bbalip.2014.07.020. PMC 4314516. PMID 25093613.
- ^ Fer, M; Dréano, Y; Lucas, D; Corcos, L; Salaün, J. P.; Berthou, F; Amet, Y (2008). "Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450". Archives of Biochemistry and Biophysics. 471 (2): 116–125. doi:10.1016/j.abb.2008.01.002. PMID 18206980.
- ^ a b Shahabi, P; Siest, G; Meyer, UA; Visvikis-Siest, S (2014). "Human cytochrome P450 epoxygenases: Variability in expression and role in inflammation-related disorders". Pharmacology & Therapeutics. 144 (2): 134–161. doi:10.1016/j.pharmthera.2014.05.011. PMID 24882266.
- ^ Frömel, T; Kohlstedt, K; Popp, R; Yin, X; Awwad, K; Barbosa-Sicard, E; Thomas, AC; Lieberz, R; Mayr, M; Fleming, I (2013). "Cytochrome P4502S1: A novel monocyte/macrophage fatty acid epoxygenase in human atherosclerotic plaques". Basic Research in Cardiology. 108 (1): 319. doi:10.1007/s00395-012-0319-8. PMID 23224081. S2CID 9158244.
- ^ Fleming, I (2014). "The pharmacology of the cytochrome P450 epoxygenase/soluble epoxide hydrolase axis in the vasculature and cardiovascular disease". Pharmacological Reviews. 66 (4): 1106–1140. doi:10.1124/pr.113.007781. PMID 25244930. S2CID 39465144.
- ^ Westphal, C; Konkel, A; Schunck, WH (2011). "CYP-eicosanoids--a new link between omega-3 fatty acids and cardiac disease?". Prostaglandins & Other Lipid Mediators. 96 (1–4): 99–108. doi:10.1016/j.prostaglandins.2011.09.001. PMID 21945326.
- ^ Matsuoka T, Narumiya S (2007). "Prostaglandin receptor signaling in disease". TheScientificWorldJournal. 7: 1329–1347. doi:10.1100/tsw.2007.182. PMC 5901339. PMID 17767353.
- ^ Thomas J, Fairclough A, Kavanagh J, Kelly AJ (2014). "Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term". The Cochrane Database of Systematic Reviews. 2014 (6): CD003101. doi:10.1002/14651858.CD003101.pub3. PMC 7138281. PMID 24941907.
- ^ Rossi A, Anzalone A, Fortuna MC, Caro G, Garelli V, Pranteda G, Carlesimo M (2016). "Multi-therapies in androgenetic alopecia: review and clinical experiences". Dermatologic Therapy. 29 (6): 424–432. doi:10.1111/dth.12390. hdl:11573/877469. PMID 27424565. S2CID 45963890.
- ^ Garza LA, Liu Y, Yang Z, Alagesan B, Lawson JA, Norberg SM, Loy DE, Zhao T, Blatt HB, Stanton DC, Carrasco L, Ahluwalia G, Fischer SM, FitzGerald GA, Cotsarelis G (2012). "Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia". Science Translational Medicine. 4 (126): 126ra34. doi:10.1126/scitranslmed.3003122. PMC 3319975. PMID 22440736.
- ^ a b Hata AN, Breyer RM (2004). "Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation". Pharmacology & Therapeutics. 103 (2): 147–166. doi:10.1016/j.pharmthera.2004.06.003. PMID 15369681.
- ^ a b Figueiredo-Pereira ME, Corwin C, Babich J (2016). "Prostaglandin J2: a potential target for halting inflammation-induced neurodegeneration". Annals of the New York Academy of Sciences. 1363 (1): 125–137. Bibcode:2016NYASA1363..125F. doi:10.1111/nyas.12987. PMC 4801700. PMID 26748744.
- ^ Hoxha M, Buccellati C, Capra V, Garella D, Cena C, Rolando B, Fruttero R, Carnevali S, Sala A, Rovati GE, Bertinaria M (2016). "In vitro pharmacological evaluation of multitarget agents for thromboxane prostanoid receptor antagonism and COX-2 inhibition" (PDF). Pharmacological Research. 103: 132–143. doi:10.1016/j.phrs.2015.11.012. hdl:2318/1551575. PMID 26621246. S2CID 12881002.
- ^ Cruz JE, Ward A, Anthony S, Chang S, Bae HB, Hermes-DeSantis ER (2016). "Evidence for the Use of Epoprostenol to Treat Raynaud's Phenomenon With or Without Digital Ulcers: A Review of the Literature". The Annals of Pharmacotherapy. 50 (12): 1060–1067. doi:10.1177/1060028016660324. PMID 27465880. S2CID 38333954.
- ^ O'Connell C, Amar D, Boucly A, Savale L, Jaïs X, Chaumais MC, Montani D, Humbert M, Simonneau G, Sitbon O (2016). "Comparative Safety and Tolerability of Prostacyclins in Pulmonary Hypertension". Drug Safety. 39 (4): 287–294. doi:10.1007/s40264-015-0365-x. PMID 26748508. S2CID 24852012.
- ^ Cacione, Daniel G.; Macedo, Cristiane R.; do Carmo Novaes, Frederico; Baptista-Silva, Jose Cc (4 May 2020). "Pharmacological treatment for Buerger's disease". The Cochrane Database of Systematic Reviews. 5 (5): CD011033. doi:10.1002/14651858.CD011033.pub4. ISSN 1469-493X. PMC 7197514. PMID 32364620.
- ^ Citterio A, Arnoldi A, Panzeri E, D'Angelo MG, Filosto M, Dilena R, Arrigoni F, Castelli M, Maghini C, Germiniasi C, Menni F, Martinuzzi A, Bresolin N, Bassi MT (2014). "Mutations in CYP2U1, DDHD2 and GBA2 genes are rare causes of complicated forms of hereditary spastic paraparesis" (PDF). Journal of Neurology. 261 (2): 373–381. doi:10.1007/s00415-013-7206-6. hdl:2434/421160. PMID 24337409. S2CID 19189811.
- ^ a b Liu M, Yokomizo T (2015). "The role of leukotrienes in allergic diseases". Allergology International. 64 (1): 17–26. doi:10.1016/j.alit.2014.09.001. PMID 25572555.
- ^ Bäck M, Dahlén SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T, Rovati GE (2011). "International Union of Basic and Clinical Pharmacology. LXXXIV: leukotriene receptor nomenclature, distribution, and pathophysiological functions". Pharmacological Reviews. 63 (3): 539–584. doi:10.1124/pr.110.004184. PMID 21771892. S2CID 5563700.
- ^ Bäck M, Powell WS, Dahlén SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T, Rovati GE (2014). "Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR Review 7". British Journal of Pharmacology. 171 (15): 3551–3574. doi:10.1111/bph.12665. PMC 4128057. PMID 24588652.
- ^ Cingi C, Muluk NB, Ipci K, Şahin E (2015). "Antileukotrienes in upper airway inflammatory diseases". Current Allergy and Asthma Reports. 15 (11): 64. doi:10.1007/s11882-015-0564-7. PMID 26385352. S2CID 38854822.
- ^ Nettis E, D'Erasmo M, Di Leo E, Calogiuri G, Montinaro V, Ferrannini A, Vacca A (2010). "The employment of leukotriene antagonists in cutaneous diseases belonging to allergological field". Mediators of Inflammation. 2010: 1–6. doi:10.1155/2010/628171. PMC 2945673. PMID 20886028.
- ^ Kanaoka Y, Maekawa A, Austen KF (2013). "Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand". The Journal of Biological Chemistry. 288 (16): 10967–10972. doi:10.1074/jbc.C113.453704. PMC 3630866. PMID 23504326.
- ^ a b c Romano M, Cianci E, Simiele F, Recchiuti A (2015). "Lipoxins and aspirin-triggered lipoxins in resolution of inflammation". European Journal of Pharmacology. 760: 49–63. doi:10.1016/j.ejphar.2015.03.083. PMID 25895638.
- ^ a b c Chiang N, Serhan CN, Dahlén SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C (2006). "The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo". Pharmacological Reviews. 58 (3): 463–487. doi:10.1124/pr.58.3.4. PMID 16968948. S2CID 6496181.
- ^ Qu Q, Xuan W, Fan GH (2015). "Roles of resolvins in the resolution of acute inflammation". Cell Biology International. 39 (1): 3–22. doi:10.1002/cbin.10345. PMID 25052386. S2CID 10160642.
- ^ Lim JY, Park CK, Hwang SW (2015). "Biological Roles of Resolvins and Related Substances in the Resolution of Pain". BioMed Research International. 2015: 830930. doi:10.1155/2015/830930. PMC 4538417. PMID 26339646.
- ^ a b Serhan CN, Chiang N, Dalli J, Levy BD (2015). "Lipid mediators in the resolution of inflammation". Cold Spring Harbor Perspectives in Biology. 7 (2): a016311. doi:10.1101/cshperspect.a016311. PMC 4315926. PMID 25359497.
- ^ Serhan CN, Chiang N (2013). "Resolution phase lipid mediators of inflammation: agonists of resolution". Current Opinion in Pharmacology. 13 (4): 632–640. doi:10.1016/j.coph.2013.05.012. PMC 3732499. PMID 23747022.
- ^ Yang L, Mäki-Petäjä K, Cheriyan J, McEniery C, Wilkinson IB (2015). "The role of epoxyeicosatrienoic acids in the cardiovascular system". British Journal of Clinical Pharmacology. 80 (1): 28–44. doi:10.1111/bcp.12603. PMC 4500322. PMID 25655310.
- ^ Clinical trial number NCT00847899 for "Evaluation of Soluble Epoxide Hydrolase (s-EH) Inhibitor in Patients With Mild to Moderate Hypertension and Impaired Glucose Tolerance" at ClinicalTrials.gov
- ^ Bos C, Richel D, Ritsema T, Peppelenbosch M, Versteeg H (2004). "Prostanoids and prostanoid receptors in signal transduction". Int J Biochem Cell Biol. 36 (7): 1187–1205. doi:10.1016/j.biocel.2003.08.006. PMID 15109566.
- ^ Samuelsson B (May 1983). "Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation". Science. 220 (4597): 568–575. Bibcode:1983Sci...220..568S. doi:10.1126/science.6301011. PMID 6301011.
- ^ Haeggström JZ, Funk CD (2011). "Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease". Chemical Reviews. 111 (10): 5866–5898. doi:10.1021/cr200246d. PMID 21936577.[permanent dead link]
- ^ Anwar Y, Sabir JS, Qureshi MI, Saini KS (2014). "5-lipoxygenase: a promising drug target against inflammatory diseases-biochemical and pharmacological regulation". Current Drug Targets. 15 (4): 410–422. doi:10.2174/1389450114666131209110745. PMID 24313690.
- ^ Kar M, Altıntoprak N, Muluk NB, Ulusoy S, Bafaqeeh SA, Cingi C (March 2016). "Antileukotrienes in adenotonsillar hypertrophy: a review of the literature". European Archives of Oto-Rhino-Laryngology. 273 (12): 4111–4117. doi:10.1007/s00405-016-3983-8. PMID 26980339. S2CID 31311115.
- ^ Oussalah A, Mayorga C, Blanca M, Barbaud A, Nakonechna A, Cernadas J, Gotua M, Brockow K, Caubet JC, Bircher A, Atanaskovic M, Demoly P, K Tanno L, Terreehorst I, Laguna JJ, Romano A, Guéant JL (April 2016). "Genetic variants associated with drugs-induced immediate hypersensitivity reactions: a PRISMA-compliant systematic review". Allergy. 71 (4): 443–462. doi:10.1111/all.12821. PMID 26678823. S2CID 13352894.
- ^ Mitchell S, Balp MM, Samuel M, McBride D, Maurer M (2015). "Systematic review of treatments for chronic spontaneous urticaria with inadequate response to licensed first-line treatments". International Journal of Dermatology. 54 (9): 1088–1104. doi:10.1111/ijd.12727. PMID 25515967. S2CID 23250789.
- ^ Wu SH, Chen XQ, Liu B, Wu HJ, Dong L (2013). "Efficacy and safety of 15(R/S)-methyl-lipoxin A(4) in topical treatment of infantile eczema". The British Journal of Dermatology. 168 (1): 172–178. doi:10.1111/j.1365-2133.2012.11177.x. PMID 22834636. S2CID 31721094.
- ^ a b Basil MC, Levy BD (2016). "Specialized pro-resolving mediators: endogenous regulators of infection and inflammation". Nature Reviews. Immunology. 16 (1): 51–67. doi:10.1038/nri.2015.4. PMC 5242505. PMID 26688348.
- ^ Feltenmark S, Gautam N, Brunnström A, Griffiths W, Backman L, Edenius C, Lindbom L, Björkholm M, Claesson HE (January 2008). "Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells". Proc. Natl. Acad. Sci. U.S.A. 105 (2): 680–685. Bibcode:2008PNAS..105..680F. doi:10.1073/pnas.0710127105. PMC 2206596. PMID 18184802.
- ^ James A, Daham K, Backman L, Brunnström A, Tingvall T, Kumlin M, Edenius C, Dahlén SE, Dahlén B, Claesson HE (2013). "The influence of aspirin on release of eoxin C4, leukotriene C4 and 15-HETE, in eosinophilic granulocytes isolated from patients with asthma". Int. Arch. Allergy Immunol. 162 (2): 135–142. doi:10.1159/000351422. PMID 23921438. S2CID 29180895.
- ^ Claesson HE (2009). "On the biosynthesis and biological role of eoxins and 15-lipoxygenase-1 in airway inflammation and Hodgkin lymphoma". Prostaglandins & Other Lipid Mediators. 89 (3–4): 120–125. doi:10.1016/j.prostaglandins.2008.12.003. PMID 19130894.
- ^ Serhan CN, Chiang N, Dalli J (2015). "The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution". Seminars in Immunology. 27 (3): 200–215. doi:10.1016/j.smim.2015.03.004. PMC 4515371. PMID 25857211.
- ^ Guichardant M, Calzada C, Bernoud-Hubac N, Lagarde M, Véricel E (2015). "Omega-3 polyunsaturated fatty acids and oxygenated metabolism in atherothrombosis". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1851 (4): 485–495. doi:10.1016/j.bbalip.2014.09.013. PMID 25263947.
- ^ Calder PC (2014). "Biomarkers of immunity and inflammation for use in nutrition interventions: International Life Sciences Institute European Branch work on selection criteria and interpretation". Endocrine, Metabolic & Immune Disorders Drug Targets. 14 (4): 236–244. doi:10.2174/1871530314666140709091650. PMID 25008763.
- ^ a b Fritsche, Kevin (August 2006). "Fatty Acids as Modulators of the Immune Response". Annual Review of Nutrition. 26: 45–73. doi:10.1146/annurev.nutr.25.050304.092610. PMID 16848700.
- ^ National Institute of Health (2005-08-01). "Omega-3 fatty acids, fish oil, alpha-linolenic acid". Archived from the original on May 3, 2006. Retrieved March 26, 2006.
- ^ Thompson, Dennis (2024-08-02). "Fish Oil Might Help High-Risk Older Adults Avoid Alzheimer's". www.healthday.com. Retrieved 2024-08-06.
- ^ Shinto, Lynne H.; Murchison, Charles F.; Silbert, Lisa C.; Dodge, Hiroko H.; Lahna, David; Rooney, William; Kaye, Jeffrey; Quinn, Joseph F.; Bowman, Gene L. (2024-08-01). "ω-3 PUFA for Secondary Prevention of White Matter Lesions and Neuronal Integrity Breakdown in Older Adults: A Randomized Clinical Trial". JAMA Network Open. 7 (8): e2426872. doi:10.1001/jamanetworkopen.2024.26872. ISSN 2574-3805. PMC 11294966. PMID 39088212.
- ^ Burr, G.O.; Burr, M.M. (1930). "On the nature and role of the fatty acids essential in nutrition". J. Biol. Chem. 86 (587): 587–621. doi:10.1016/S0021-9258(20)78929-5.
- ^ Bergström, S.; Danielsson, H.; Samuelsson, B. (1964). "The enzymatic formation of prostaglandin E2 from arachidonic acid". Biochim. Biophys. Acta. 90 (207): 207–210. doi:10.1016/0304-4165(64)90145-x. PMID 14201168.
- ^ Vane, J. R. (June 23, 1971). "Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs". Nature New Biology. 231 (25): 232–235. doi:10.1038/newbio231232a0. PMID 5284360.
External links
[edit]- Eicosanoids at the U.S. National Library of Medicine Medical Subject Headings (MeSH)





