Mothers against decapentaplegic homolog 4
SMAD4, also called SMAD family member 4, Mothers against decapentaplegic homolog 4, or DPC4 (Deleted in Pancreatic Cancer-4) is a highly conserved protein present in all metazoans. It belongs to the SMAD family of transcription factor proteins, which act as mediators of TGF-β signal transduction. The TGFβ family of cytokines regulates critical processes during the lifecycle of metazoans, with important roles during embryo development, tissue homeostasis, regeneration, and immune regulation.[5]
SMAD 4 belongs to the co-SMAD group (common mediator SMAD), the second class of the SMAD family. SMAD4 is the only known co-SMAD in most metazoans. It also belongs to the Darwin family of proteins that modulate members of the TGFβ protein superfamily, a family of proteins that all play a role in the regulation of cellular responses. Mammalian SMAD4 is a homolog of the Drosophila protein "Mothers against decapentaplegic" named Medea.[6]
SMAD4 interacts with R-Smads, such as SMAD2, SMAD3, SMAD1, SMAD5 and SMAD9 (also called SMAD8) to form heterotrimeric complexes. Transcriptional coregulators, such as WWTR1 (TAZ) interact with SMADs to promote their function. Once in the nucleus, the complex of SMAD4 and two R-SMADS binds to DNA and regulates the expression of different genes depending on the cellular context.[6] Intracellular reactions involving SMAD4 are triggered by the binding, on the surface of the cells, of growth factors from the TGFβ family. The sequence of intracellular reactions involving SMADS is called the SMAD pathway or the transforming growth factor beta (TGF-β) pathway since the sequence starts with the recognition of TGF-β by cells.
Gene
[edit]In mammals, SMAD4 is coded by a gene located on chromosome 18. In humans, the SMAD4 gene contains 54 829 base pairs and is located from pair n° 51,030,212 to pair 51,085,041 in the region 21.1 of the chromosome 18.[7][8]

Protein
[edit]SMAD4 is a 552 amino-acid polypeptide with a molecular weight of 60.439 Da. SMAD4 has two functional domains known as MH1 and MH2.
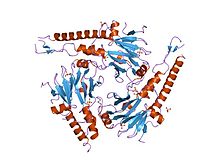
The complex of two SMAD3 (or of two SMAD2) and one SMAD4 binds directly to DNA though interactions of their MH1 domains. These complexes are recruited to sites throughout the genome by cell lineage-defining transcription factors (LDTFs) that determine the context-dependent nature of TGF-β action. Early insights into the DNA binding specificity of Smad proteins came from oligonucleotide binding screens, which identified the palindromic duplex 5'–GTCTAGAC–3' as a high affinity binding sequence for SMAD3 and SMAD4 MH1 domains.[9] Other motifs have also been identified in promoters and enhancers. These additional sites contain the CAGCC motif and the GGC(GC)|(CG) consensus sequences, the latter also known as 5GC sites.[10] The 5GC-motifs are highly represented as clusters of sites, in SMAD-bound regions genome-wide. These clusters can also contain CAG(AC)|(CC) sites. SMAD3/SMAD4 complex also binds to the TPA-responsive gene promoter elements, which have the sequence motif TGAGTCAG.[11]
Structures
[edit]MH1 domain complexes with DNA motifs
[edit]The first structure of SMAD4 bound to DNA was the complex with the palindromic GTCTAGAC motif.[12] Recently, the structures of SMAD4 MH1 domain bound to several 5GC motifs have also been determined. In all complexes, the interaction with the DNA involves a conserved β-hairpin present in the MH1 domain. The hairpin is partially flexible in solution and its high degree of conformational flexibility allows recognition of the different 5-bp sequences. Efficient interactions with GC-sites occur only if a G nucleotide is located deep in the major grove, and establishes hydrogen bonds with the guanidinium group of Arg81. This interaction facilitates a complementary surface contact between the Smad DNA-binding hairpin and the major groove of the DNA. Other direct interactions involve Lys88 and Gln83. The X-ray crystal structure of the Trichoplax adhaerens SMAD4 MH1 domains bound to the GGCGC motif indicates a high conservation of this interaction in metazoans.[10]



MH2 domain complexes
[edit]The MH2 domain, corresponding to the C-terminus, is responsible for receptor recognition and association with other SMADs. It interacts with the R-SMADS MH2 domain and forms heterodimers and heterotrimers. Some tumor mutations detected in SMAD4 enhance interactions between the MH1 and MH2 domains.[13]
Nomenclature and origin of name
[edit]SMADs are highly conserved across species, especially in the N terminal MH1 domain and the C terminal MH2 domain. The SMAD proteins are homologs of both the Drosophila protein MAD and the C. elegans protein SMA. The name is a combination of the two. During Drosophila research, it was found that a mutation in the gene MAD in the mother repressed the gene decapentaplegic in the embryo. The phrase "Mothers against" was added, since mothers often form organizations opposing various issues, e.g. Mothers Against Drunk Driving (MADD), reflecting "the maternal-effect enhancement of dpp";[14] and based on a tradition of unusual naming within the research community.[15] SMAD4 is also known as DPC4, JIP or MADH4.
Function and action mechanism
[edit]SMAD4 is a protein defined as an essential effector in the SMAD pathway. SMAD4 serves as a mediator between extracellular growth factors from the TGFβ family and genes inside the cell nucleus. The abbreviation co in co-SMAD stands for common mediator. SMAD4 is also defined as a signal transducer.
In the TGF-β pathway, TGF-β dimers are recognized by a transmembrane receptor, known as type II receptor. Once the type II receptor is activated by the binding of TGF-β, it phosphorylates a type I receptor. Type I receptor is also a cell surface receptor. This receptor then phosphorylates intracellular receptor regulated SMADS (R-SMADS) such as SMAD2 or SMAD3. The phosphorylated R-SMADS then bind to SMAD4. The R-SMADs-SMAD4 association is a heteromeric complex. This complex is going to move from the cytoplasm to the nucleus: it is the translocation. SMAD4 may form heterotrimeric, heterohexameric or heterodimeric complexes with R-SMADS.
SMAD4 is a substrate of the Erk/MAPK kinase[16] and GSK3.[17] The FGF (Fibroblast Growth Factor) pathway stimulation leads to Smad4 phosphorylation by Erk of the canonical MAPK site located at Threonine 277. This phosphorylation event has a dual effect on Smad4 activity. First, it allows Smad4 to reach its peak of transcriptional activity by activating a growth factor-regulated transcription activation domain located in the Smad4 linker region, SAD (Smad-Activation Domain).[18] Second, MAPK primes Smad4 for GSK3-mediated phosphorylations that cause transcriptional inhibition and also generate a phosphodegron used as a docking site by the ubiquitin E3 ligase Beta-transducin Repeat Containing (beta-TrCP) that polyubiquitinates Smad4 and targets it for degradation in the proteasome.[19] Smad4 GSK3 phosphorylations have been proposed to regulate the protein stability during pancreatic and colon cancer progression.[20]
In the nucleus the heteromeric complex binds promoters and interact with transcriptional activators. SMAD3/SMAD4 complexes can directly bind the SBE. These associations are weak and require additional transcription factors such as members of the AP-1 family, TFE3 and FoxG1 to regulate gene expression.[21]
Many TGFβ ligands use this pathway and subsequently SMAD4 is involved in many cell functions such as differentiation, apoptosis, gastrulation, embryonic development and the cell cycle.
Clinical significance
[edit]Genetic experiments such as gene knockout (KO), which consist in modifying or inactivating a gene, can be carried out in order to see the effects of a dysfunctional SMAD 4 on the study organism. Experiments are often conducted in the house mouse (Mus musculus).
It has been shown that, in mouse KO of SMAD4, the granulosa cells, which secrete hormones and growth factors during the oocyte development, undergo premature luteinization and express lower levels of follicle-stimulating hormone receptors (FSHR) and higher levels of luteinizing hormone receptors (LHR). This may be due in part to impairment of bone morphogenetic protein-7 effects as BMP-7 uses the SMAD4 signaling pathway.[22][23]
Deletions in the genes coding for SMAD1 and SMAD5 have also been linked to metastasic granulosa cell tumors in mice.[24]
SMAD4, is often found mutated in many cancers. The mutation can be inherited or acquired during an individual's lifetime. If inherited, the mutation affects both somatic cells and cells of the reproductive organs. If the SMAD 4 mutation is acquired, it will only exist in certain somatic cells. Indeed, SMAD 4 is not synthesized by all cells. The protein is present in skin, pancreatic, colon, uterus and epithelial cells. It is also produced by fibroblasts. The functional SMAD 4 participates in the regulation of the TGF-β signal transduction pathway, which negatively regulates growth of epithelial cells and the extracellular matrix (ECM). When the structure of SMAD 4 is altered, expression of the genes involved in cell growth is no longer regulated and cell proliferation can go on without any inhibition. The important number of cell divisions leads to the forming of tumors and then to multiploid colorectal cancer and pancreatic carcinoma. It is found inactivated in at least 50% of pancreatic cancers.[25]
Somatic mutations found in human cancers of the MH1 domain of SMAD 4 have been shown to inhibit the DNA-binding function of this domain.
SMAD 4 is also found mutated in the autosomal dominant disease juvenile polyposis syndrome (JPS). JPS is characterized by hamartomatous polyps in the gastrointestinal (GI) tract. These polyps are usually benign, however they are at greater risk of developing gastrointestinal cancers, in particular colon cancer. Around 60 mutations causing JPS have been identified. They have been linked to the production of a smaller SMAD 4, with missing domains that prevent the protein from binding to R-SMADS and forming heteromeric complexes.[8]
Mutations in SMAD4 (mostly substitutions) can cause Myhre syndrome, a rare inherited disorder characterized by mental disabilities, short stature, unusual facial features, and various bone abnormalities.[26][27]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000141646 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000024515 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Massagué J (October 2012). "TGFβ signalling in context". Nature Reviews. Molecular Cell Biology. 13 (10): 616–630. doi:10.1038/nrm3434. PMC 4027049. PMID 22992590.
- ^ a b Massagué J (1998). "TGF-beta signal transduction". Annual Review of Biochemistry. 67 (1): 753–791. doi:10.1146/annurev.biochem.67.1.753. PMID 9759503.
- ^ "SMAD4 SMAD family member 4". Entrez Gene.
- ^ a b "SMAD 4". The Genetics Home Reference Website.
- ^ Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, et al. (March 1998). "Human Smad3 and Smad4 are sequence-specific transcription activators". Molecular Cell. 1 (4): 611–617. doi:10.1016/s1097-2765(00)80061-1. PMID 9660945.
- ^ a b Martin-Malpartida P, Batet M, Kaczmarska Z, Freier R, Gomes T, Aragón E, et al. (December 2017). "Structural basis for genome wide recognition of 5-bp GC motifs by SMAD transcription factors". Nature Communications. 8 (1): 2070. Bibcode:2017NatCo...8.2070M. doi:10.1038/s41467-017-02054-6. PMC 5727232. PMID 29234012.
- ^ Zhang Y, Feng XH, Derynck R (August 1998). "Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription". Nature. 394 (6696): 909–913. Bibcode:1998Natur.394..909Z. doi:10.1038/29814. PMID 9732876. S2CID 4393852.
- ^ Baburajendran N, Jauch R, Tan CY, Narasimhan K, Kolatkar PR (October 2011). "Structural basis for the cooperative DNA recognition by Smad4 MH1 dimers". Nucleic Acids Research. 39 (18): 8213–8222. doi:10.1093/nar/gkr500. PMC 3185416. PMID 21724602.
- ^ Hata A, Lo RS, Wotton D, Lagna G, Massagué J (July 1997). "Mutations increasing autoinhibition inactivate tumour suppressors Smad2 and Smad4". Nature. 388 (6637): 82–87. Bibcode:1997Natur.388R..82H. doi:10.1038/40424. PMID 9214507. S2CID 4407819.
- ^ Sekelsky JJ, Newfeld SJ, Raftery LA, Chartoff EH, Gelbart WM (March 1995). "Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster". Genetics. 139 (3): 1347–1358. doi:10.1093/genetics/139.3.1347. PMC 1206461. PMID 7768443.
- ^ White M (26 September 2014). "Sonic Hedgehog, DICER, and the Problem With Naming Genes". Pacific Standard.
- ^ Roelen BA, Cohen OS, Raychowdhury MK, Chadee DN, Zhang Y, Kyriakis JM, et al. (October 2003). "Phosphorylation of threonine 276 in Smad4 is involved in transforming growth factor-beta-induced nuclear accumulation". American Journal of Physiology. Cell Physiology. 285 (4): C823 – C830. doi:10.1152/ajpcell.00053.2003. PMID 12801888. S2CID 9326115.
- ^ Demagny H, Araki T, De Robertis EM (October 2014). "The tumor suppressor Smad4/DPC4 is regulated by phosphorylations that integrate FGF, Wnt, and TGF-β signaling". Cell Reports. 9 (2): 688–700. doi:10.1016/j.celrep.2014.09.020. PMID 25373906.
- ^ de Caestecker MP, Yahata T, Wang D, Parks WT, Huang S, Hill CS, et al. (January 2000). "The Smad4 activation domain (SAD) is a proline-rich, p300-dependent transcriptional activation domain". The Journal of Biological Chemistry. 275 (3): 2115–2122. doi:10.1074/jbc.275.3.2115. PMID 10636916.
- ^ Demagny H, De Robertis EM (March 2016). "Smad4/DPC4: A barrier against tumor progression driven by RTK/Ras/Erk and Wnt/GSK3 signaling". Molecular & Cellular Oncology. 3 (2): e989133. doi:10.4161/23723556.2014.989133. PMC 4905428. PMID 27308623.
- ^ Demagny H, De Robertis EM (January 2016). "Point mutations in the tumor suppressor Smad4/DPC4 enhance its phosphorylation by GSK3 and reversibly inactivate TGF-β signaling". Molecular & Cellular Oncology. 3 (1): e1025181. doi:10.1080/23723556.2015.1025181. PMC 4845174. PMID 27308538.
- ^ Inman GJ (February 2005). "Linking Smads and transcriptional activation". The Biochemical Journal. 386 (Pt 1): e1 – e3. doi:10.1042/bj20042133. PMC 1134782. PMID 15702493.
- ^ Shi J, Yoshino O, Osuga Y, Nishii O, Yano T, Taketani Y (March 2010). "Bone morphogenetic protein 7 (BMP-7) increases the expression of follicle-stimulating hormone (FSH) receptor in human granulosa cells". Fertility and Sterility. 93 (4): 1273–1279. doi:10.1016/j.fertnstert.2008.11.014. PMID 19108831.
- ^ Pangas SA, Li X, Robertson EJ, Matzuk MM (June 2006). "Premature luteinization and cumulus cell defects in ovarian-specific Smad4 knockout mice". Molecular Endocrinology. 20 (6): 1406–1422. doi:10.1210/me.2005-0462. PMID 16513794.
- ^ Middlebrook BS, Eldin K, Li X, Shivasankaran S, Pangas SA (December 2009). "Smad1-Smad5 ovarian conditional knockout mice develop a disease profile similar to the juvenile form of human granulosa cell tumors". Endocrinology. 150 (12): 5208–5217. doi:10.1210/en.2009-0644. PMC 2819741. PMID 19819941.
- ^ Cotran RS, Kumar V, Fausto N, Robbins SL, Abbas AK (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. ISBN 0-7216-0187-1.
- ^ "Growth-Mental Deficiency Syndrome of Myhre". National Organization for rare disorders. Archived from the original on 2 April 2015. Retrieved 26 March 2015.
- ^ Caputo V, Bocchinfuso G, Castori M, Traversa A, Pizzuti A, Stella L, et al. (July 2014). "Novel SMAD4 mutation causing Myhre syndrome". American Journal of Medical Genetics. Part A. 164A (7): 1835–1840. doi:10.1002/ajmg.a.36544. PMID 24715504. S2CID 5294309.
Further reading
[edit]- Miyazono K (2000). "TGF-beta signaling by Smad proteins". Cytokine & Growth Factor Reviews. 11 (1–2): 15–22. doi:10.1016/S1359-6101(99)00025-8. PMID 10708949.
- Wrana JL, Attisano L (2000). "The Smad pathway". Cytokine & Growth Factor Reviews. 11 (1–2): 5–13. doi:10.1016/S1359-6101(99)00024-6. PMID 10708948.
- Verschueren K, Huylebroeck D (2000). "Remarkable versatility of Smad proteins in the nucleus of transforming growth factor-beta activated cells". Cytokine & Growth Factor Reviews. 10 (3–4): 187–199. doi:10.1016/S1359-6101(99)00012-X. PMID 10647776.
- Massagué J (1998). "TGF-beta signal transduction". Annual Review of Biochemistry. 67: 753–791. doi:10.1146/annurev.biochem.67.1.753. PMID 9759503.
- Klein-Scory S, Zapatka M, Eilert-Micus C, Hoppe S, Schwarz E, Schmiegel W, et al. (November 2007). "High-level inducible Smad4-reexpression in the cervical cancer cell line C4-II is associated with a gene expression profile that predicts a preferential role of Smad4 in extracellular matrix composition". BMC Cancer. 7: 209. doi:10.1186/1471-2407-7-209. PMC 2186346. PMID 17997817.
- Kalo E, Buganim Y, Shapira KE, Besserglick H, Goldfinger N, Weisz L, et al. (December 2007). "Mutant p53 attenuates the SMAD-dependent transforming growth factor beta1 (TGF-beta1) signaling pathway by repressing the expression of TGF-beta receptor type II". Molecular and Cellular Biology. 27 (23): 8228–8242. doi:10.1128/MCB.00374-07. PMC 2169171. PMID 17875924.
- Aretz S, Stienen D, Uhlhaas S, Stolte M, Entius MM, Loff S, et al. (November 2007). "High proportion of large genomic deletions and a genotype phenotype update in 80 unrelated families with juvenile polyposis syndrome". Journal of Medical Genetics. 44 (11): 702–709. doi:10.1136/jmg.2007.052506. PMC 2752176. PMID 17873119.
- Ali S, Cohen C, Little JV, Sequeira JH, Mosunjac MB, Siddiqui MT (October 2007). "The utility of SMAD4 as a diagnostic immunohistochemical marker for pancreatic adenocarcinoma, and its expression in other solid tumors". Diagnostic Cytopathology. 35 (10): 644–648. doi:10.1002/dc.20715. PMID 17854080. S2CID 36682992.
- Milet J, Dehais V, Bourgain C, Jouanolle AM, Mosser A, Perrin M, et al. (October 2007). "Common variants in the BMP2, BMP4, and HJV genes of the hepcidin regulation pathway modulate HFE hemochromatosis penetrance". American Journal of Human Genetics. 81 (4): 799–807. doi:10.1086/520001. PMC 2227929. PMID 17847004.
- Salek C, Benesova L, Zavoral M, Nosek V, Kasperova L, Ryska M, et al. (July 2007). "Evaluation of clinical relevance of examining K-ras, p16 and p53 mutations along with allelic losses at 9p and 18q in EUS-guided fine needle aspiration samples of patients with chronic pancreatitis and pancreatic cancer". World Journal of Gastroenterology. 13 (27): 3714–3720. doi:10.3748/wjg.v13.i27.3714. PMC 4250643. PMID 17659731.
- Sebestyén A, Hajdu M, Kis L, Barna G, Kopper L (September 2007). "Smad4-independent, PP2A-dependent apoptotic effect of exogenous transforming growth factor beta 1 in lymphoma cells". Experimental Cell Research. 313 (15): 3167–3174. doi:10.1016/j.yexcr.2007.05.028. PMID 17643425.
- Levy L, Howell M, Das D, Harkin S, Episkopou V, Hill CS (September 2007). "Arkadia activates Smad3/Smad4-dependent transcription by triggering signal-induced SnoN degradation". Molecular and Cellular Biology. 27 (17): 6068–6083. doi:10.1128/MCB.00664-07. PMC 1952153. PMID 17591695.
- Grijelmo C, Rodrigue C, Svrcek M, Bruyneel E, Hendrix A, de Wever O, et al. (August 2007). "Proinvasive activity of BMP-7 through SMAD4/src-independent and ERK/Rac/JNK-dependent signaling pathways in colon cancer cells". Cellular Signalling. 19 (8): 1722–1732. doi:10.1016/j.cellsig.2007.03.008. PMID 17478078.
- Sonegawa H, Nukui T, Li DW, Takaishi M, Sakaguchi M, Huh NH (July 2007). "Involvement of deterioration in S100C/A11-mediated pathway in resistance of human squamous cancer cell lines to TGFbeta-induced growth suppression". Journal of Molecular Medicine. 85 (7): 753–762. doi:10.1007/s00109-007-0180-7. PMID 17476473. S2CID 15667203.
- Sheikh AA, Vimalachandran D, Thompson CC, Jenkins RE, Nedjadi T, Shekouh A, et al. (June 2007). "The expression of S100A8 in pancreatic cancer-associated monocytes is associated with the Smad4 status of pancreatic cancer cells". Proteomics. 7 (11): 1929–1940. doi:10.1002/pmic.200700072. PMID 17469085. S2CID 35648264.
- Popović Hadzija M, Korolija M, Jakić Razumović J, Pavković P, Hadzija M, Kapitanović S (April 2007). "K-ras and Dpc4 mutations in chronic pancreatitis: case series". Croatian Medical Journal. 48 (2): 218–224. PMC 2080529. PMID 17436386.
- Losi L, Bouzourene H, Benhattar J (May 2007). "Loss of Smad4 expression predicts liver metastasis in human colorectal cancer". Oncology Reports. 17 (5): 1095–1099. doi:10.3892/or.17.5.1095. PMID 17390050.
- Karlsson G, Blank U, Moody JL, Ehinger M, Singbrant S, Deng CX, et al. (March 2007). "Smad4 is critical for self-renewal of hematopoietic stem cells". The Journal of Experimental Medicine. 204 (3): 467–474. doi:10.1084/jem.20060465. PMC 2137898. PMID 17353364.
- Takano S, Kanai F, Jazag A, Ijichi H, Yao J, Ogawa H, et al. (March 2007). "Smad4 is essential for down-regulation of E-cadherin induced by TGF-beta in pancreatic cancer cell line PANC-1". Journal of Biochemistry. 141 (3): 345–351. doi:10.1093/jb/mvm039. PMID 17301079.