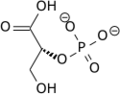
Phosphoglycerate mutase

| Phosphoglycerate mutase family | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||
| Symbol | PGAM | ||||||||||
| Pfam | PF00300 | ||||||||||
| InterPro | IPR013078 | ||||||||||
| PROSITE | PDOC00158 | ||||||||||
| SCOP2 | 3pgm / SCOPe / SUPFAM | ||||||||||
| |||||||||||
| phosphoglycerate mutase 1 (brain) | |||||||
|---|---|---|---|---|---|---|---|
 | |||||||
| Identifiers | |||||||
| Symbol | PGAM1 | ||||||
| Alt. symbols | PGAMA | ||||||
| NCBI gene | 5223 | ||||||
| HGNC | 8888 | ||||||
| OMIM | 172250 | ||||||
| RefSeq | NM_002629 | ||||||
| UniProt | P18669 | ||||||
| Other data | |||||||
| EC number | 5.4.2.11 | ||||||
| Locus | Chr. 10 q25.3 | ||||||
| |||||||
| phosphoglycerate mutase 2 (muscle) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | PGAM2 | ||||||
| NCBI gene | 5224 | ||||||
| HGNC | 8889 | ||||||
| OMIM | 261670 | ||||||
| RefSeq | NM_000290 | ||||||
| UniProt | P15259 | ||||||
| Other data | |||||||
| EC number | 5.4.2.11 | ||||||
| Locus | Chr. 7 p13-p12 | ||||||
| |||||||
- This enzyme is not to be confused with Bisphosphoglycerate mutase which catalyzes the conversion of 1,3-bisphosphoglycerate to 2,3-bisphosphoglycerate.
Phosphoglycerate mutase (PGM) is any enzyme that catalyzes step 8 of glycolysis - the internal transfer of a phosphate group from C-3 to C-2 which results in the conversion of 3-phosphoglycerate (3PG) to 2-phosphoglycerate (2PG) through a 2,3-bisphosphoglycerate intermediate. These enzymes are categorized into the two distinct classes of either cofactor-dependent (dPGM) or cofactor-independent (iPGM).[1] The dPGM enzyme (EC 5.4.2.11) is composed of approximately 250 amino acids and is found in all vertebrates as well as in some invertebrates, fungi, and bacteria. The iPGM (EC 5.4.2.12) class is found in all plants and algae as well as in some invertebrate, fungi, and Gram-positive bacteria.[2] This class of PGM enzyme shares the same superfamily as alkaline phosphatase.[3]
Mechanism
[edit]PGM is an isomerase enzyme, effectively transferring a phosphate group (PO43−) from the C-3 carbon of 3-phosphoglycerate to the C-2 carbon forming 2-phosphoglycerate. There are a total of three reactions dPGM can catalyze: a mutase reaction resulting in the conversion of 3PG to 2PG and vice versa,[4][5] a phosphatase reaction creating phosphoglycerate from 2,3-bisphosphoglycerate,[6][7] and a synthase reaction producing 2,3-bisphosphoglycerate from 1,3-bisphosphoglycerate similar to the enzyme bisphosphoglycerate mutase[citation needed]. Kinetic and structural studies have provided evidence that indicate dPGM and bisphosphoglycerate mutase are paralogous structures.[6] Both enzymes are contained in the superfamily that also contains the phosphatase portion of phosphofructokinase 2 and prostatic acid phosphatase.[8]
The catalyzed mutase reaction involves two separate phosphoryl groups and the ending phosphate on the 2-carbon is not the same phosphate removed from the 3-carbon.
In the cofactor-dependent enzyme's initial state, the active site contains a phosphohistidine complex formed by phosphorylation of a specific histidine residue.[9] When 3-phosphoglycerate enters the active site, the phosphohistidine complex is positioned as to facilitate transfer of phosphate from enzyme to substrate C-2 creating a 2,3-bisphosphoglycerate intermediate.
Dephosphorylation of the enzyme histidine actuates a local allosteric change in enzyme configuration which now aligns the substrates 3-C phosphate group with enzyme active site histidine and facilitates phosphate transfer returning the enzyme to its initial phosphorylated state and releasing product 2-phosphoglycerate. 2,3-bisphosphoglycerate is required a cofactor for dPGM. In contrast, the iPGM class is independent of 2,3-bisphosphoglycerate and catalyzes the intramolecular transfer of the phosphate group on monophosphoglycerates using a phosphoserineintermediate.[10]
Reaction summary
[edit]3PG + P-Enzyme → 2,3BPG + Enzyme → 2PG + P-Enzyme
3-phosphoglycerate intermediate 2-phosphoglycerate
ΔG°′=+1.1kcal/mol
Isozymes
[edit]Phosphoglycerate mutase exists primarily as a dimer of two either identical or closely related subunits of about 32kDa. The enzyme is found in organisms as simple as yeast through Homo sapiens and its structure is highly conserved throughout. (Yeast PGM≈74% conserved vs mammal form).
In mammals, the enzyme subunits appear to be either a muscle-derived form (m-type) or other tissue (b-type for brain where the b-isozyme was originally isolated). Existing as a dimer, the enzyme then has 3 isozymes depending on which subunit forms makeup the whole molecule (mm, bb or mb). The mm-type is found mainly in smooth muscle almost exclusively. The mb-isozyme is found in cardiac and skeletal muscle and the bb-type is found in the rest of tissues.[11] While all three isozymes may be found in any tissue, the above distributions are based on prevalence in each.
Interactive pathway map
[edit]Click on genes, proteins and metabolites below to link to respective articles.[§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "GlycolysisGluconeogenesis_WP534".
Regulation
[edit]Phosphoglycerate mutase has a small positive Gibbs free energy and this reaction proceeds easily in both directions. Since it is a reversible reaction, it is not the site of major regulation mechanisms or regulation schemes for the glycolytic pathway.
Anionic molecules such as vanadate,[12] acetate, chloride ion, phosphate, 2-phosphoglycolate, and N-[tris(hydroxymethyl)methyl-2-amino]ethanesulfonate are known inhibitors of the mutase activity of dPGM. Studies have shown dPGM to be sensitive to changes in ionic concentration, where increasing concentrations of salts result in the activation of the enzyme's phosphatase activity while inhibiting its mutase activity. Certain salts, such as KCl, are known to be competitive inhibitors in respect to 2-phosphoglycerate and mutase activity.[13] Both phosphate and 2-phosphoglycolate are competitive inhibitors of mutase activity in respect to the substrates 2-phosphoglycerate and 2,3-bisphosphoglycerate.[14]
Clinical significance
[edit]In humans the PGAM2 gene which encodes this enzyme is located on the short arm of chromosome 7.
Deficiency of phosphoglycerate mutase causes glycogen storage disease type X, a rare autosomal recessive genetic disorder with symptoms ranging from mild to moderate; is not thought life-threatening and can be managed with changes in lifestyle.[citation needed] This presents as a metabolic myopathy and is one of the many forms of syndromes formerly referred to as muscular dystrophy.[citation needed] PGAM1 deficiency affects the liver, while PGAM2 deficiency affects the muscle.
Onset is generally noted as childhood to early adult though some who may be mildly affected by the disorder may not know they have it. Patients with PGAM deficiency are usually asymptomatic, except when they engage in brief, strenuous efforts which may trigger myalgias, cramps, muscle necrosis and myoglobinuria.[15] An unusual pathologic feature of PGAM deficiency is the association with tubular aggregates. The symptoms are an intolerance to physical exertion or activity, cramps and muscle pain. Permanent weakness is rare. The disease is not progressive and has an excellent prognosis.[citation needed]
Human proteins containing this domain
[edit]BPGM; PFKFB1; PFKFB2; PFKFB3; PFKFB4; PGAM1; PGAM2; PGAM4; PGAM5; STS1; UBASH3A;
References
[edit]- ^ Johnsen, U; Schönheit, P (September 2007). "Characterization of cofactor-dependent and cofactor-independent phosphoglycerate mutases from Archaea". Extremophiles: Life Under Extreme Conditions. 11 (5): 647–57. doi:10.1007/s00792-007-0094-x. PMID 17576516. S2CID 5836321.
- ^ Jedrzejas, MJ (2000). "Structure, function, and evolution of phosphoglycerate mutases: comparison with fructose-2,6-bisphosphatase, acid phosphatase, and alkaline phosphatase". Progress in Biophysics and Molecular Biology. 73 (2–4): 263–87. doi:10.1016/s0079-6107(00)00007-9. PMID 10958932.
- ^ Galperin, MY; Bairoch, A; Koonin, EV (August 1998). "A superfamily of metalloenzymes unifies phosphopentomutase and cofactor-independent phosphoglycerate mutase with alkaline phosphatases and sulfatases". Protein Science. 7 (8): 1829–35. doi:10.1002/pro.5560070819. PMC 2144072. PMID 10082381.
- ^ Sasaki, R; Utsumi, S; Sugimoto, E; Chiba, H (15 July 1976). "Subunit structure and multifunctional properties of yeast phosphoglyceromutase". European Journal of Biochemistry. 66 (3): 523–33. doi:10.1111/j.1432-1033.1976.tb10578.x. PMID 182494.
- ^ Rose, ZB; Dube, S (25 August 1976). "Rates of phosphorylation and dephosphorylation of phosphoglycerate mutase and bisphosphoglycerate synthase". The Journal of Biological Chemistry. 251 (16): 4817–22. doi:10.1016/S0021-9258(17)33188-5. PMID 8447.
- ^ a b Rose, ZB; Dube, S (10 December 1978). "Phosphoglycerate mutase. Kinetics and effects of salts on the mutase and bisphosphoglycerate phosphatase activities of the enzyme from chicken breast muscle". The Journal of Biological Chemistry. 253 (23): 8583–92. doi:10.1016/S0021-9258(17)34332-6. PMID 213437.
- ^ Sasaki, R; Hirose, M; Sugimoto, E; Chiba, H (10 March 1971). "Studies on a role of the 2,3-diphosphoglycerate phosphatase activity in the yeast phosphoglycerate mutase reaction". Biochimica et Biophysica Acta (BBA) - Enzymology. 227 (3): 595–607. doi:10.1016/0005-2744(71)90010-6. PMID 4328052.
- ^ Wang, Y; Wei, Z; Liu, L; Cheng, Z; Lin, Y; Ji, F; Gong, W (17 June 2005). "Crystal structure of human B-type phosphoglycerate mutase bound with citrate". Biochemical and Biophysical Research Communications. 331 (4): 1207–15. doi:10.1016/j.bbrc.2005.03.243. PMID 15883004.
- ^ Britton, HG; Clarke, JB (March 1969). "The mechanism of the phosphoglycerate mutase reaction". The Biochemical Journal. 112 (1): 10P – 11P. doi:10.1042/bj1120010pb. PMC 1187664. PMID 5774486.
- ^ Jedrzejas, MJ; Chander, M; Setlow, P; Krishnasamy, G (28 July 2000). "Mechanism of catalysis of the cofactor-independent phosphoglycerate mutase from Bacillus stearothermophilus. Crystal structure of the complex with 2-phosphoglycerate". The Journal of Biological Chemistry. 275 (30): 23146–53. doi:10.1074/jbc.m002544200. PMID 10764795.
- ^ Omenn, GS; Cheung, SC (May 1974). "Phosphoglycerate mutase isozyme marker for tissue differentiation in man". American Journal of Human Genetics. 26 (3): 393–9. PMC 1762627. PMID 4827367.
- ^ Song, L; Xu, Z; Yu, X (August 2007). "Molecular cloning and characterization of a phosphoglycerate mutase gene from Clonorchis sinensis". Parasitology Research. 101 (3): 709–14. doi:10.1007/s00436-007-0540-9. PMID 17468884. S2CID 104159.
- ^ Grisolia, S; Tecson, J (11 January 1967). "Mercury-induced reversible increase in 2,3-diphosphoglycerate phosphatase and concomitant decrease in mutase activity of animal phosphoglycerate mutases". Biochimica et Biophysica Acta (BBA) - Enzymology. 132 (1): 56–67. doi:10.1016/0005-2744(67)90191-x. PMID 4291574.
- ^ Grisolia, S; Cleland, WW (March 1968). "Influence of salt, substrate, and cofactor concentrations on the kinetic and mechanistic behavior of phosphoglycerate mutase". Biochemistry. 7 (3): 1115–21. doi:10.1021/bi00843a032. PMID 5690561.
- ^ Salameh J, Goyal N, Choudry R, Camelo-Piragua S, Chong PS (July 2012). "Phosphoglycerate mutase deficiency with tubular aggregates in a patient from panama" (PDF). Muscle Nerve. 47 (1): 138–40. doi:10.1002/mus.23527. hdl:2027.42/95158. PMID 23169535. S2CID 34151935.
External links
[edit]- Phosphoglycerate+Mutase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- PDBe-KB provides an overview of all the structure information available in the PDB for Human Phosphoglycerate mutase 1